Effect of substituents at the C3´, C3´N, C10 and C2-meta-benzoate positions of taxane derivatives on their activity against resistant cancer cells
- PMID: 38870637
- PMCID: PMC11257372
- DOI: 10.1016/j.taap.2024.116993
Effect of substituents at the C3´, C3´N, C10 and C2-meta-benzoate positions of taxane derivatives on their activity against resistant cancer cells
Abstract
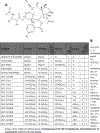
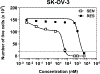
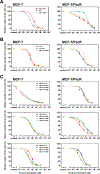
We tested the effect of substituents at the (1) C3´, C3´N, (2) C10, and (3) C2-meta-benzoate positions of taxane derivatives on their activity against sensitive versus counterpart paclitaxel-resistant breast (MCF-7) and ovarian (SK-OV-3) cancer cells. We found that (1) non-aromatic groups at both C3´ and C3´N positions, when compared with phenyl groups at the same positions of a taxane derivative, significantly reduced the resistance of ABCB1 expressing MCF-7/PacR and SK-OV-3/PacR cancer cells. This is, at least in the case of the SB-T-1216 series, accompanied by an ineffective decrease of intracellular levels in MCF-7/PacR cells. The low binding affinity of SB-T-1216 in the ABCB1 binding cavity can elucidate these effects. (2) Cyclopropanecarbonyl group at the C10 position, when compared with the H atom, seems to increase the potency and capability of the derivative in overcoming paclitaxel resistance in both models. (3) Derivatives with fluorine and methyl substituents at the C2-meta-benzoate position were variously potent against sensitive and resistant cancer cells. All C2 derivatives were less capable of overcoming acquired resistance to paclitaxel in vitro than non-substituted analogs. Notably, fluorine derivatives SB-T-121205 and 121,206 were more potent against sensitive and resistant SK-OV-3 cells, and derivatives SB-T-121405 and 121,406 were more potent against sensitive and resistant MCF-7 cells. (4) The various structure-activity relationships of SB-T derivatives observed in two cell line models known to express ABCB1 favor their complex interaction not based solely on ABCB1.
Keywords: C10 taxane derivatives; C2 taxane derivatives; C3´ and C3´N taxane derivatives; Resistant breast cancer cells; Resistant ovarian cancer cells.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
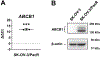


Similar articles
-
Substituents at the C3' and C3'N positions are critical for taxanes to overcome acquired resistance of cancer cells to paclitaxel.Toxicol Appl Pharmacol. 2018 May 15;347:79-91. doi: 10.1016/j.taap.2018.04.002. Epub 2018 Apr 4. Toxicol Appl Pharmacol. 2018. PMID: 29625142 Free PMC article.
-
Taxane monotherapy regimens for the treatment of recurrent epithelial ovarian cancer.Cochrane Database Syst Rev. 2022 Jul 12;7(7):CD008766. doi: 10.1002/14651858.CD008766.pub3. Cochrane Database Syst Rev. 2022. PMID: 35866378 Free PMC article.
-
Design, synthesis and SAR study of Fluorine-containing 3rd-generation taxoids.Bioorg Chem. 2022 Feb;119:105578. doi: 10.1016/j.bioorg.2021.105578. Epub 2021 Dec 23. Bioorg Chem. 2022. PMID: 34979464 Free PMC article.
-
Topotecan, pegylated liposomal doxorubicin hydrochloride and paclitaxel for second-line or subsequent treatment of advanced ovarian cancer: a systematic review and economic evaluation.Health Technol Assess. 2006 Mar;10(9):1-132. iii-iv. doi: 10.3310/hta10090. Health Technol Assess. 2006. PMID: 16545208
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of topotecan for ovarian cancer.Health Technol Assess. 2001;5(28):1-110. doi: 10.3310/hta5280. Health Technol Assess. 2001. PMID: 11701100
Cited by
-
Cyclosporine A Decreased Paclitaxel Resistance in Prostate Cancer Cells by Inhibiting MTDH Expression.Am J Mens Health. 2025 Jan-Feb;19(1):15579883241310834. doi: 10.1177/15579883241310834. Am J Mens Health. 2025. PMID: 39744936 Free PMC article.
References
-
- Alli E, Yang JM, Ford JM, and Hait WN (2007). Reversal of stathmin-mediated resistance to paclitaxel and vinblastine in human breast carcinoma cells. Mol Pharmacol 71, 1233–1240. - PubMed
-
- Brooks TA, Minderman H, O’Loughlin KL, Pera P, Ojima I, Baer MR, and Bernacki RJ (2003). Taxane-based reversal agents modulate drug resistance mediated by P-glycoprotein, multidrug resistance protein, and breast cancer resistance protein. Mol Cancer Ther 2, 1195–1205. - PubMed
-
- Cermak V, Dostal V, Jelinek M, Libusova L, Kovar J, Rosel D, and Brabek J (2020). Microtubule-targeting agents and their impact on cancer treatment. Eur J Cell Biol 99, 151075. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

