Clinical outcome assessments of disease burden and progression in late-onset GM2 gangliosidoses
- PMID: 38870773
- PMCID: PMC11317923
- DOI: 10.1016/j.ymgme.2024.108512
Clinical outcome assessments of disease burden and progression in late-onset GM2 gangliosidoses
Abstract
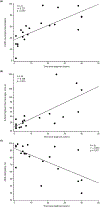
The late-onset GM2 gangliosidoses, comprising late-onset Tay-Sachs and Sandhoff diseases, are rare, slowly progressive, neurogenetic disorders primarily characterized by neurogenic weakness, ataxia, and dysarthria. The aim of this longitudinal study was to characterize the natural history of late-onset GM2 gangliosidoses using a number of clinical outcome assessments to measure different aspects of disease burden and progression over time, including neurological, functional, and quality of life, to inform the design of future clinical interventional trials. Patients attending the United States National Tay-Sachs & Allied Diseases Family Conference between 2015 and 2019 underwent annual clinical outcome assessments. Currently, there are no clinical outcome assessments validated to assess late-onset GM2 gangliosidoses; therefore, instruments used or designed for diseases with similar features, or to address various aspects of the clinical presentations, were used. Clinical outcome assessments included the Friedreich's Ataxia Rating Scale, the 9-Hole Peg Test, and the Assessment of Intelligibility of Dysarthric Speech. Twenty-three patients participated in at least one meeting visit (late-onset Tay-Sachs, n = 19; late-onset Sandhoff, n = 4). Patients had high disease burden at baseline, and scores for the different clinical outcome assessments were generally lower than would be expected for the general population. Longitudinal analyses showed slow, but statistically significant, neurological progression as evidenced by worsening scores on the 9-Hole Peg Test (2.68%/year, 95% CI: 0.13-5.29; p = 0.04) and the Friedreich's Ataxia Rating Scale neurological examination (1.31 points/year, 95% CI: 0.26-2.35; p = 0.02). Time since diagnosis to study entry correlated with worsening scores on the 9-Hole Peg Test (r = 0.728; p < 0.001), Friedreich's Ataxia Rating Scale neurological examination (r = 0.727; p < 0.001), and Assessment of Intelligibility of Dysarthric Speech intelligibility (r = -0.654; p = 0.001). In summary, patients with late-onset GM2 gangliosidoses had high disease burden and slow disease progression. Several clinical outcome assessments suitable for clinical trials showed only small changes and standardized effect sizes (change/standard deviation of change) over 4 years. These longitudinal natural history study results illustrate the challenge of identifying responsive endpoints for clinical trials in rare, slowly progressive, neurogenerative disorders where arguably the treatment goal is to halt or decrease the rate of decline rather than improve clinical status. Furthermore, powering such a study would require a large sample size and/or a long study duration, neither of which is an attractive option for an ultra-rare disease with no available treatment. These findings support the development of potentially more sensitive late-onset GM2 gangliosidoses-specific rating instruments and/or surrogate endpoints for use in future clinical trials.
Keywords: Clinical outcome assessments; Late-onset Sandhoff disease; Late-onset Tay–Sachs disease; Lysosomal storage disease; Natural history study; Neurodegenerative disorder.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest JK, CR, PM, and AH are employees of Sanofi and may hold shares and/or stock options in the company. GFC was a former employee of Sanofi through 2016, and since 2019, is the owner of Gerald Cox Rare Care Consulting, LLC and a member of the NTSAD Board of Directors. CDS and FE are neurologists volunteering at the NTSAD. CDS has provided scientific advisory for SwanBio Therapeutics and his institution has received research funding from Sanofi for a study of video oculography in late-onset GM2 gangliosidoses. CDS has received financial support from SwanBio Therapeutics, Encora Therapeutics, Sanofi, Biogen and Biohaven for the conduct of clinical trials. CDS has received honoraria from the International Parkinson's and Movement Disorders Society, the American Academy of Neurology/Continuum and Oakstone CME for the production of educational material. CDS has received grant support from the National Institutes of HealthK23 NS118045-03. FE is co-principal investigator of a gene therapy trial in GM2 sponsored by Sio Pharmaceuticals and site principal investigator of a trial on late onset GM2 sponsored by Sanofi. HL was a former employee of NYU Langone Hospital in New York City, and currently is employed by Ultragenyx and volunteers at NTSAD. JMJ and CT are employees of the Intramural Research Program (IRP) of the National Human Genome Research Institute (NHGRI), National Institutes of Health (NIH), Bethesda, Maryland. RK is an employee of IQVIA, which received funding from Sanofi to conduct qualitative research to develop the GM2 gangliosidoses conceptual framework, and to support selection of the COA instruments used in this study. GFC, FE, CDS, HL and CT assessed the patients and did not receive funding for their participation.
Figures

References
-
- Mahuran DJ, Biochemical consequences of mutations causing the GM2 gangliosidoses Biochimica et biophysica acta 1455 (1999) 105–138. - PubMed
-
- Gravel RA, Kaback MM, Proia RL, Sandhoff K, Suzuki K, Suzuki K, The GM2 Gangliosidoses, in: Valle DL, Antonarakis S, Ballabio A, Beaudet AL, Mitchell GA (Eds.), The Online Metabolic and Molecular Bases of Inherited Disease, McGraw-Hill Education, New York, NY, 2019.
-
- Neudorfer O, Pastores GM, Zeng BJ, Gianutsos J, Zaroff CM, Kolodny EH, Late-onset Tay-Sachs disease: phenotypic characterization and genotypic correlations in 21 affected patients Genetics in medicine : official journal of the American College of Medical Genetics 7 (2005) 119–123. - PubMed
-
- Masingue M, Dufour L, Lenglet T, Saleille L, Goizet C, Ayrignac X, Ory-Magne F, Barth M, Lamari F, Mandia D, Caillaud C, Nadjar Y, Natural History of Adult Patients with GM2 Gangliosidosis Annals of neurology 87 (2020) 609–617. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

