Activation of Nrf2 inhibits atherosclerosis in ApoE-/- mice through suppressing endothelial cell inflammation and lipid peroxidation
- PMID: 38870781
- PMCID: PMC11247299
- DOI: 10.1016/j.redox.2024.103229
Activation of Nrf2 inhibits atherosclerosis in ApoE-/- mice through suppressing endothelial cell inflammation and lipid peroxidation
Abstract
Background: Nuclear erythroid 2-related factor 2 (Nrf2), a transcription factor, is critically involved in the regulation of oxidative stress and inflammation. However, the role of endothelial Nrf2 in atherogenesis has yet to be defined. In addition, how endothelial Nrf2 is activated and whether Nrf2 can be targeted for the prevention and treatment of atherosclerosis is not explored.
Methods: RNA-sequencing and single-cell RNA sequencing analysis of mouse atherosclerotic aortas were used to identify the differentially expressed genes. In vivo endothelial cell (EC)-specific activation of Nrf2 was achieved by injecting adeno-associated viruses into ApoE-/- mice, while EC-specific knockdown of Nrf2 was generated in Cdh5CreCas9floxed-stopApoE-/- mice.
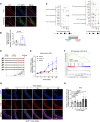
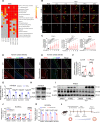
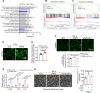
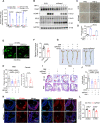
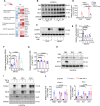
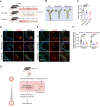
Results: Endothelial inflammation appeared as early as on day 3 after feeding of a high cholesterol diet (HCD) in ApoE-/- mice, as reflected by mRNA levels, immunostaining and global mRNA profiling, while the immunosignal of the end-product of lipid peroxidation (LPO), 4-hydroxynonenal (4-HNE), started to increase on day 10. TNF-α, 4-HNE, and erastin (LPO inducer), activated Nrf2 signaling in human ECs by increasing the mRNA and protein expression of Nrf2 target genes. Knockdown of endothelial Nrf2 resulted in augmented endothelial inflammation and LPO, and accelerated atherosclerosis in Cdh5CreCas9floxed-stopApoE-/- mice. By contrast, both EC-specific and pharmacological activation of Nrf2 inhibited endothelial inflammation, LPO, and atherogenesis.
Conclusions: Upon HCD feeding in ApoE-/- mice, endothelial inflammation is an earliest event, followed by the appearance of LPO. EC-specific activation of Nrf2 inhibits atherosclerosis while EC-specific knockdown of Nrf2 results in the opposite effect. Pharmacological activators of endothelial Nrf2 may represent a novel therapeutic strategy for the treatment of atherosclerosis.
Keywords: Atherosclerosis; Endothelial cells; Inflammation; Lipid peroxidation; Nuclear erythroid 2-related factor 2.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that there is no conflict of interest regarding the publication of this paper.
Figures

References
-
- Libby P. The changing landscape of atherosclerosis. Nature. 2021;592:524–533. - PubMed
-
- Libby P., Theroux P. Pathophysiology of coronary artery disease. Circulation. 2005;111:3481–3488. - PubMed
-
- Ridker P.M., Everett B.M., Thuren T., MacFadyen J.G., Chang W.H., Ballantyne C., et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N. Engl. J. Med. 2017;377:1119–1131. - PubMed
-
- Ridker P.M., Rane M. Interleukin-6 signaling and anti-interleukin-6 therapeutics in cardiovascular disease. Circ. Res. 2021;128:1728–1746. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

