Nutrient acquisition strategies by gut microbes
- PMID: 38870902
- PMCID: PMC11178278
- DOI: 10.1016/j.chom.2024.05.011
Nutrient acquisition strategies by gut microbes
Abstract
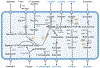
The composition and function of the gut microbiota are intimately tied to nutrient acquisition strategies and metabolism, with significant implications for host health. Both dietary and host-intrinsic factors influence community structure and the basic modes of bacterial energy metabolism. The intestinal tract is rich in carbon and nitrogen sources; however, limited access to oxygen restricts energy-generating reactions to fermentation. By contrast, increased availability of electron acceptors during episodes of intestinal inflammation results in phylum-level changes in gut microbiota composition, suggesting that bacterial energy metabolism is a key driver of gut microbiota function. In this review article, we will illustrate diverse examples of microbial nutrient acquisition strategies in the context of habitat filters and anatomical location and the central role of energy metabolism in shaping metabolic strategies to support bacterial growth in the mammalian gut.
Keywords: bacterial metabolism; fermentation; gut microbiota; respiration.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures




Similar articles
-
[Advances in host-microbe metabolic axis].Wei Sheng Wu Xue Bao. 2017 Feb 4;57(2):161-9. Wei Sheng Wu Xue Bao. 2017. PMID: 29750478 Review. Chinese.
-
Bacterial interactions on nutrient-rich surfaces in the gut lumen.Infect Immun. 2024 Sep 10;92(9):e0048023. doi: 10.1128/iai.00480-23. Epub 2024 Mar 20. Infect Immun. 2024. PMID: 38506518 Free PMC article. Review.
-
Seasonal Dietary Shifts Alter the Gut Microbiota of Avivorous Bats: Implication for Adaptation to Energy Harvest and Nutritional Utilization.mSphere. 2021 Aug 25;6(4):e0046721. doi: 10.1128/mSphere.00467-21. Epub 2021 Aug 4. mSphere. 2021. PMID: 34346703 Free PMC article.
-
A review of metabolic potential of human gut microbiome in human nutrition.Arch Microbiol. 2018 Mar;200(2):203-217. doi: 10.1007/s00203-017-1459-x. Epub 2017 Nov 29. Arch Microbiol. 2018. PMID: 29188341 Review.
-
Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite.J Nutr. 2017 May;147(5):727-745. doi: 10.3945/jn.116.240481. Epub 2017 Mar 29. J Nutr. 2017. PMID: 28356427 Review.
Cited by
-
Revisiting nitrogen assimilation strategies in the mammalian gut: lessons from Enterobacteriaceae as pathobiont models and a challenge to the limitation paradigm.Arch Microbiol. 2025 Jul 28;207(9):203. doi: 10.1007/s00203-025-04404-1. Arch Microbiol. 2025. PMID: 40719856 Free PMC article. Review.
-
Chemical Composition of Cynanchum auriculatum Royle Ex Wight and Its Potential Role in Ameliorating Colitis.Food Sci Nutr. 2025 Jan 19;13(1):e4764. doi: 10.1002/fsn3.4764. eCollection 2025 Jan. Food Sci Nutr. 2025. PMID: 39830908 Free PMC article.
-
Effect of diet-induced low-grade, chronic inflammation on susceptibility of broilers to Salmonella colonization and pathogenicity.Poult Sci. 2025 Aug;104(8):105303. doi: 10.1016/j.psj.2025.105303. Epub 2025 May 21. Poult Sci. 2025. PMID: 40480134 Free PMC article.
-
Microbial metabolism of host-derived antioxidants.Curr Opin Chem Biol. 2025 Feb;84:102565. doi: 10.1016/j.cbpa.2024.102565. Epub 2024 Dec 24. Curr Opin Chem Biol. 2025. PMID: 39721219 Review.
-
Interbacterial warfare in the human gut: insights from Bacteroidales' perspective.Gut Microbes. 2025 Dec;17(1):2473522. doi: 10.1080/19490976.2025.2473522. Epub 2025 Mar 4. Gut Microbes. 2025. PMID: 40038576 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

