Initiation of metformin in early pregnancy results in fetal bioaccumulation, growth restriction, and renal dysmorphology in a primate model
- PMID: 38871238
- PMCID: PMC11344684
- DOI: 10.1016/j.ajog.2024.06.002
Initiation of metformin in early pregnancy results in fetal bioaccumulation, growth restriction, and renal dysmorphology in a primate model
Abstract
Background: In recent years, pragmatic metformin use in pregnancy has stretched to include prediabetes mellitus, type 2 diabetes mellitus, gestational diabetes mellitus, and (most recently) preeclampsia. However, with its expanded use, concerns of unintended harm have been raised.
Objective: This study developed an experimental primate model and applied ultrahigh performance liquid chromatography coupled to triple-quadrupole mass spectrometry for direct quantitation of maternal and fetal tissue metformin levels with detailed fetal biometry and histopathology.
Study design: Within 30 days of confirmed conception (defined as early pregnancy), 13 time-bred (timed-mated breeding) Rhesus dams with pregnancies designated for fetal necropsy were initiated on twice-daily human dose-equivalent 10 mg/kg metformin or vehicle control. Pregnant dams were maintained as pairs and fed either a control chow or 36% fat Western-style diet. Metformin or placebo vehicle control was delivered in various treats while the animals were separated via a slide. A cesarean delivery was performed at gestational day 145, and amniotic fluid and blood were collected, and the fetus and placenta were delivered. The fetus was immediately necropsied by trained primate center personnel. All fetal organs were dissected, measured, sectioned, and processed per clinical standards. Fluid and tissue metformin levels were assayed using validated ultrahigh performance liquid chromatography coupled to triple-quadrupole mass spectrometry in selected reaction monitoring against standard curves.
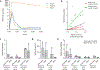
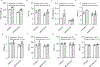
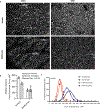
Results: Among 13 pregnancies at gestational day 145 with fetal necropsy, 1 dam and its fetal tissues had detectable metformin levels despite being allocated to the vehicle control group (>1 μmol metformin/kg maternal weight or fetal or placental tissue), whereas a second fetus allocated to the vehicle control group had severe fetal growth restriction (birthweight of 248.32 g [<1%]) and was suspected of having a fetal congenital condition. After excluding these 2 fetal pregnancies from further analyses, 11 fetuses from dams initiated on either vehicle control (n=4: 3 female and 1 male fetuses) or 10 mg/kg metformin (n=7: 5 female and 2 male fetuses) were available for analyses. Among dams initiated on metformin at gestational day 30 (regardless of maternal diet), significant bioaccumulation within the fetal kidney (0.78-6.06 μmol/kg; mean of 2.48 μmol/kg), liver (0.16-0.73 μmol/kg; mean of 0.38 μmol/kg), fetal gut (0.28-1.22 μmol/kg; mean of 0.70 μmol/kg), amniotic fluid (0.43-3.33 μmol/L; mean of 1.88 μmol/L), placenta (0.16-1.00 μmol/kg; mean of 0.50 μmol/kg), fetal serum (0.00-0.66 μmol/L; mean of 0.23 μmol/L), and fetal urine (4.10-174.10 μmol/L; mean of 38.5 μmol/L) was observed, with fetal levels near biomolar equivalent to maternal levels (maternal serum: 0.18-0.86 μmol/L [mean of 0.46 μmol/L]; maternal urine: 42.60-254.00 μmol/L [mean of 149.30 μmol/L]). Western-style diet feeding neither accelerated nor reduced metformin bioaccumulations in maternal or fetal serum, urine, amniotic fluid, placenta, or fetal tissues. In these 11 animals, fetal bioaccumulation of metformin was associated with less fetal skeletal muscle (57% lower cross-sectional area of gastrocnemius) and decreased liver, heart, and retroperitoneal fat masses (P<.05), collectively driving lower delivery weight (P<.0001) without changing the crown-rump length. Sagittal sections of fetal kidneys demonstrated delayed maturation, with disorganized glomerular generations and increased cortical thickness. This renal dysmorphology was not accompanied by structural or functional changes indicative of renal insufficiency.
Conclusion: Our study demonstrates fetal bioaccumulation of metformin with associated fetal growth restriction and renal dysmorphology after maternal initiation of the drug within 30 days of conception in primates. Given these results and the prevalence of metformin use during pregnancy, additional investigation of any potential immediate and enduring effects of prenatal metformin use is warranted.
Keywords: fetal bioaccumulation; fetal programming; insulin resistance; metformin use; obesity.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
COMPETING INTERESTS STATEMENT
No authors have any conflicts of interest to declare.
Figures



Similar articles
-
Maternal but not fetoplacental health can be improved by metformin in a murine diet-induced model of maternal obesity and glucose intolerance.J Physiol. 2022 Feb;600(4):903-919. doi: 10.1113/JP281902. Epub 2021 Sep 29. J Physiol. 2022. PMID: 34505282 Free PMC article.
-
L-Citrulline Supplementation Enhances Fetal Growth and Protein Synthesis in Rats with Intrauterine Growth Restriction.J Nutr. 2016 Mar;146(3):532-41. doi: 10.3945/jn.115.221267. Epub 2016 Feb 10. J Nutr. 2016. PMID: 26865647
-
Maternal exercise in rats upregulates the placental insulin-like growth factor system with diet- and sex-specific responses: minimal effects in mothers born growth restricted.J Physiol. 2018 Dec;596(23):5947-5964. doi: 10.1113/JP275758. Epub 2018 Jul 26. J Physiol. 2018. PMID: 29953638 Free PMC article.
-
Obstetrical complications associated with abnormal maternal serum markers analytes.J Obstet Gynaecol Can. 2008 Oct;30(10):918-932. doi: 10.1016/S1701-2163(16)32973-5. J Obstet Gynaecol Can. 2008. PMID: 19038077 Review. English, French.
-
Nutritionally mediated placental growth restriction in the growing adolescent: consequences for the fetus.Biol Reprod. 2004 Oct;71(4):1055-62. doi: 10.1095/biolreprod.104.030965. Epub 2004 Jun 16. Biol Reprod. 2004. PMID: 15201203 Review.
Cited by
-
Effect of in utero metformin exposure in gestational diabetes mellitus on infant mesenchymal stem cell metabolism.Am J Physiol Endocrinol Metab. 2025 Apr 1;328(4):E567-E578. doi: 10.1152/ajpendo.00428.2024. Epub 2025 Mar 12. Am J Physiol Endocrinol Metab. 2025. PMID: 40072921 Free PMC article.
-
Preexisting Diabetes and Pregnancy: An Endocrine Society and European Society of Endocrinology Joint Clinical Practice Guideline.J Clin Endocrinol Metab. 2025 Aug 7;110(9):2405-2452. doi: 10.1210/clinem/dgaf288. J Clin Endocrinol Metab. 2025. PMID: 40652453 Free PMC article.
-
Long-term effects of metformin on offspring health: A review of current evidence and future directions.Diabetes Obes Metab. 2025 Jun;27 Suppl 3(Suppl 3):48-63. doi: 10.1111/dom.16418. Epub 2025 May 6. Diabetes Obes Metab. 2025. PMID: 40326052 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

