Analyses of 1236 genotyped primary ciliary dyskinesia individuals identify regional clusters of distinct DNA variants and significant genotype-phenotype correlations
- PMID: 38871375
- PMCID: PMC11306806
- DOI: 10.1183/13993003.01769-2023
Analyses of 1236 genotyped primary ciliary dyskinesia individuals identify regional clusters of distinct DNA variants and significant genotype-phenotype correlations
Abstract
Background: Primary ciliary dyskinesia (PCD) represents a group of rare hereditary disorders characterised by deficient ciliary airway clearance that can be associated with laterality defects. We aimed to describe the underlying gene defects, geographical differences in genotypes and their relationship to diagnostic findings and clinical phenotypes.
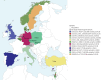
Methods: Genetic variants and clinical findings (age, sex, body mass index, laterality defects, forced expiratory volume in 1 s (FEV1)) were collected from 19 countries using the European Reference Network's ERN-LUNG international PCD Registry. Genetic data were evaluated according to American College of Medical Genetics and Genomics guidelines. We assessed regional distribution of implicated genes and genetic variants as well as genotype correlations with laterality defects and FEV1.
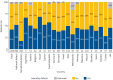
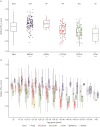
Results: The study included 1236 individuals carrying 908 distinct pathogenic DNA variants in 46 PCD genes. We found considerable variation in the distribution of PCD genotypes across countries due to the presence of distinct founder variants. The prevalence of PCD genotypes associated with pathognomonic ultrastructural defects (mean 72%, range 47-100%) and laterality defects (mean 42%, range 28-69%) varied widely among countries. The prevalence of laterality defects was significantly lower in PCD individuals without pathognomonic ciliary ultrastructure defects (18%). The PCD cohort had a reduced median FEV1 z-score (-1.66). Median FEV1 z-scores were significantly lower in CCNO (-3.26), CCDC39 (-2.49) and CCDC40 (-2.96) variant groups, while the FEV1 z-score reductions were significantly milder in DNAH11 (-0.83) and ODAD1 (-0.85) variant groups compared to the whole PCD cohort.
Conclusion: This unprecedented multinational dataset of DNA variants and information on their distribution across countries facilitates interpretation of the genetic epidemiology of PCD and indicates that the genetic variant can predict diagnostic and phenotypic features such as the course of lung function.
Copyright ©The authors 2024.
Conflict of interest statement
Conflict of interest: The authors have no potential conflicts of interest to disclose.
Figures



Comment in
-
Not all are the same: the power of registries in defining genotype-phenotype relationships in primary ciliary dyskinesia.Eur Respir J. 2024 Aug 8;64(2):2401026. doi: 10.1183/13993003.01026-2024. Print 2024 Aug. Eur Respir J. 2024. PMID: 39117425 Free PMC article. No abstract available.
References
-
- Pennekamp P, Raidt J, Wohlgemuth K, et al. . Primary ciliary dyskinesia. In: Wagner TOF, Humbert M, Wijsenbeek M, et al., eds. Rare Diseases of the Respiratory System. Sheffield, European Respiratory Society, 2023; pp. 118–134.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
