Neoadjuvant camrelizumab (an anti-PD-1 antibody) plus chemotherapy or apatinib (a VEGFR-2 inhibitor) for initially unresectable stage II-III non-small-cell lung cancer: a multicentre, two-arm, phase 2 exploratory study
- PMID: 38871690
- PMCID: PMC11176298
- DOI: 10.1038/s41392-024-01861-w
Neoadjuvant camrelizumab (an anti-PD-1 antibody) plus chemotherapy or apatinib (a VEGFR-2 inhibitor) for initially unresectable stage II-III non-small-cell lung cancer: a multicentre, two-arm, phase 2 exploratory study
Abstract
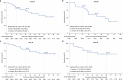
This multicentre, two-arm, phase 2 study aimed to explore the efficacy and safety of neoadjuvant camrelizumab plus chemotherapy or apatinib in patients with initially unresectable stage II-III non-small-cell lung cancer (NSCLC). Eligible patients regardless of PD-L1 expression received neoadjuvant camrelizumab 200 mg and platinum-doublet chemotherapy every 3 weeks (arm A) or those with PD-L1-positive tumors received neoadjuvant camrelizumab and apatinib 250 mg once daily (arm B), for 2-4 cycles, followed by surgery. The primary endpoint was major pathological response (MPR) rate. Thirty patients in arm A and 21 in arm B were enrolled. Surgery rates were 50.0% (15/30) in arm A and 42.9% (9/21) in arm B, with all patients achieving R0 resections. Of these patients, the MPR and pathological complete response rates were both 20.0% (95% CI 4.3-48.1) in arm A and were 55.6% (95% CI 21.2-86.3) and 11.1% (95% CI 0.3-48.2) in arm B, respectively. The corresponding objective response rates were 33.3% (95% CI 11.8-61.6) and 55.6% (95% CI 21.2-86.3). With a median follow-up of 22.4 months (95% CI 19.0-26.0), the median event-free survival was not reached (NR; 95% CI 13.6-NR) in arm A and 16.8 months (95% CI 8.6-NR) in arm B. Grade 3 or above treatment-related adverse events occurred in eight (26.7%) patients in arm A and three (14.3%) in arm B. Biomarker analysis showed baseline TYROBP expression was predictive of treatment response in arm B. Neoadjuvant camrelizumab plus chemotherapy or apatinib exhibits preliminary efficacy and manageable toxicity in patients with initially unresectable stage II-III NSCLC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Han B, et al. Cancer incidence and mortality in China, 2022. J. Natl Cancer Cent. 2024;4:47–53. doi: 10.1016/j.jncc.2024.01.006. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

