MRI-based microthrombi detection in stroke with polydopamine iron oxide
- PMID: 38871729
- PMCID: PMC11176332
- DOI: 10.1038/s41467-024-49480-x
MRI-based microthrombi detection in stroke with polydopamine iron oxide
Abstract
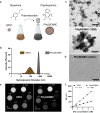
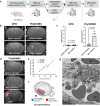
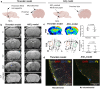
In acute ischemic stroke, even when successful recanalization is obtained, downstream microcirculation may still be obstructed by microvascular thrombosis, which is associated with compromised brain reperfusion and cognitive decline. Identifying these microthrombi through non-invasive methods remains challenging. We developed the PHySIOMIC (Polydopamine Hybridized Self-assembled Iron Oxide Mussel Inspired Clusters), a MRI-based contrast agent that unmasks these microthrombi. In a mouse model of thromboembolic ischemic stroke, our findings demonstrate that the PHySIOMIC generate a distinct hypointense signal on T2*-weighted MRI in the presence of microthrombi, that correlates with the lesion areas observed 24 hours post-stroke. Our microfluidic studies reveal the role of fibrinogen in the protein corona for the thrombosis targeting properties. Finally, we observe the biodegradation and biocompatibility of these particles. This work demonstrates that the PHySIOMIC particles offer an innovative and valuable tool for non-invasive in vivo diagnosis and monitoring of microthrombi, using MRI during ischemic stroke.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: S.M.d.L., C.J., D.V., T.B., and M.G. have filed a patent application (WO2023007002A1) for the use of PHySIOMIC as a contrast agent to reveal microthrombi in ischemic stroke. The other authors declare that they have no competing interests.
Figures




References
-
- Molina CA, et al. REVASCAT: a randomized trial of revascularization with SOLITAIRE FR device vs. best medical therapy in the treatment of acute stroke due to anterior circulation large vessel occlusion presenting within eight-hours of symptom onset. Int. J. Stroke. 2015;10:619–626. doi: 10.1111/ijs.12157. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

