Study on intestinal parasitic infections and gut microbiota in cancer patients at a tertiary teaching hospital in Malaysia
- PMID: 38871760
- PMCID: PMC11176305
- DOI: 10.1038/s41598-024-59969-6
Study on intestinal parasitic infections and gut microbiota in cancer patients at a tertiary teaching hospital in Malaysia
Abstract
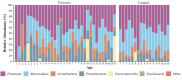
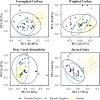
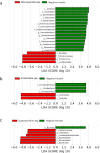
Intestinal parasitic infections (IPIs) can lead to significant morbidity and mortality in cancer patients. While they are unlikely to cause severe disease and are self-limiting in healthy individuals, cancer patients are especially susceptible to opportunistic parasitic infections. The gut microbiota plays a crucial role in various aspects of health, including immune regulation and metabolic processes. Parasites occupy the same environment as bacteria in the gut. Recent research suggests intestinal parasites can disrupt the normal balance of the gut microbiota. However, there is limited understanding of this co-infection dynamic among cancer patients in Malaysia. A study was conducted to determine the prevalence and relationship between intestinal parasites and gut microbiota composition in cancer patients. Stool samples from 134 cancer patients undergoing active treatment or newly diagnosed were collected and examined for the presence of intestinal parasites and gut microbiota composition. The study also involved 17 healthy individuals for comparison and control. Sequencing with 16S RNA at the V3-V4 region was used to determine the gut microbial composition between infected and non-infected cancer patients and healthy control subjects. The overall prevalence of IPIs among cancer patients was found to be 32.8%. Microsporidia spp. Accounted for the highest percentage at 20.1%, followed by Entamoeba spp. (3.7%), Cryptosporidium spp. (3.0%), Cyclospora spp. (2.2%), and Ascaris lumbricoides (0.8%). None of the health control subjects tested positive for intestinal parasites. The sequencing data analysis revealed that the gut microbiota diversity and composition were significantly different in cancer patients than in healthy controls (p < 0.001). A significant dissimilarity was observed in the bacterial composition between parasite-infected and non-infected patients based on Bray-Curtis (p = 0.041) and Jaccard (p = 0.021) measurements. Bacteria from the genus Enterococcus were enriched in the parasite-infected groups, while Faecalibacterium prausnitzii reduced compared to non-infected and control groups. Further analysis between different IPIs and non-infected individuals demonstrated a noteworthy variation in Entamoeba-infected (unweighted UniFrac: p = 0.008), Cryptosporidium-infected (Bray-Curtis: p = 0.034) and microsporidia-infected (unweighted: p = 0.026; weighted: p = 0.019; Jaccard: p = 0.031) samples. No significant dissimilarity was observed between Cyclospora-infected groups and non-infected groups. Specifically, patients infected with Cryptosporidium and Entamoeba showed increased obligate anaerobic bacteria. Clostridiales were enriched with Entamoeba infections, whereas those from Coriobacteriales decreased. Bacteroidales and Clostridium were found in higher abundance in the gut microbiota with Cryptosporidium infection, while Bacillales decreased. Additionally, bacteria from the genus Enterococcus were enriched in microsporidia-infected patients. In contrast, bacteria from the Clostridiales order, Faecalibacterium, Parabacteroides, Collinsella, Ruminococcus, and Sporosarcina decreased compared to the non-infected groups. These findings underscore the importance of understanding and managing the interactions between intestinal parasites and gut microbiota for improved outcomes in cancer patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Exposure to Parasitic Protists and Helminths Changes the Intestinal Community Structure of Bacterial Communities in a Cohort of Mother-Child Binomials from a Semirural Setting in Mexico.mSphere. 2021 Aug 25;6(4):e0008321. doi: 10.1128/mSphere.00083-21. Epub 2021 Aug 18. mSphere. 2021. PMID: 34406855 Free PMC article.
-
Intestinal protozoan infections shape fecal bacterial microbiota in children from Guinea-Bissau.PLoS Negl Trop Dis. 2021 Mar 3;15(3):e0009232. doi: 10.1371/journal.pntd.0009232. eCollection 2021 Mar. PLoS Negl Trop Dis. 2021. PMID: 33657123 Free PMC article.
-
Determining intestinal parasitic infections (IPIs) in inmates from Kajang Prison, Selangor, Malaysia for improved prison management.BMC Infect Dis. 2015 Oct 29;15:467. doi: 10.1186/s12879-015-1178-3. BMC Infect Dis. 2015. PMID: 26511347 Free PMC article.
-
Prevalence of intestinal parasitic infections among communities living in different habitats and its comparison with one hundred and one studies conducted over the past 42 years (1970 to 2013) in Malaysia.Trop Biomed. 2014 Jun;31(2):190-206. Trop Biomed. 2014. PMID: 25134888 Review.
-
Interaction between Intestinal Parasites and the Gut Microbiota: Implications for the Intestinal Immune Response and Host Defence.Pathogens. 2024 Jul 23;13(8):608. doi: 10.3390/pathogens13080608. Pathogens. 2024. PMID: 39204209 Free PMC article. Review.
Cited by
-
Global prevalence of intestinal parasites in cancer patients: a systematic review and meta-analysis.BMC Infect Dis. 2025 Jul 1;25(1):815. doi: 10.1186/s12879-025-11207-8. BMC Infect Dis. 2025. PMID: 40596954 Free PMC article.
-
Characterisation of gut microbiota in Malaysian cancer patients using V3-V4 region of 16S rRNA gene sequencing.Sci Rep. 2025 Jul 1;15(1):21723. doi: 10.1038/s41598-025-06983-x. Sci Rep. 2025. PMID: 40596568 Free PMC article.
References
-
- Wong LW, et al. Human intestinal parasitic infection: A narrative review on global prevalence and epidemiological insights on preventive, therapeutic and diagnostic strategies for future perspectives. Expert Rev. Gastroenterol. Hepatol. 2020;14:1093–1105. doi: 10.1080/17474124.2020.1806711. - DOI - PubMed
-
- Rodríguez-Pérez EG, et al. Opportunistic intestinal parasites in immunocompromised patients from a tertiary hospital in Monterrey, Mexico. Le Infezioni Med. 2019;27:168–174. - PubMed
-
- Angela R, Suresh K. Microsporidia in stools from cancer patients. Res. J. Med. Sci. 2007;2(1):88–90.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

