Transmissible long-term neuroprotective and pro-cognitive effects of 1-42 beta-amyloid with A2T icelandic mutation in an Alzheimer's disease mouse model
- PMID: 38871852
- PMCID: PMC11609088
- DOI: 10.1038/s41380-024-02611-8
Transmissible long-term neuroprotective and pro-cognitive effects of 1-42 beta-amyloid with A2T icelandic mutation in an Alzheimer's disease mouse model
Abstract
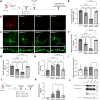
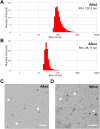
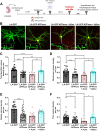
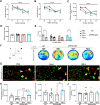
The amyloid cascade hypothesis assumes that the development of Alzheimer's disease (AD) is driven by a self-perpetuating cycle, in which β-amyloid (Aβ) accumulation leads to Tau pathology and neuronal damages. A particular mutation (A673T) of the amyloid precursor protein (APP) was identified among Icelandic population. It provides a protective effect against Alzheimer- and age-related cognitive decline. This APP mutation leads to the reduced production of Aβ with A2T (position in peptide sequence) change (Aβice). In addition, Aβice has the capacity to form protective heterodimers in association with wild-type Aβ. Despite the emerging interest in Aβice during the last decade, the impact of Aβice on events associated with the amyloid cascade has never been reported. First, the effects of Aβice were evaluated in vitro by electrophysiology on hippocampal slices and by studying synapse morphology in cortical neurons. We showed that Aβice protects against endogenous Aβ-mediated synaptotoxicity. Second, as several studies have outlined that a single intracerebral administration of Aβ can worsen Aβ deposition and cognitive functions several months after the inoculation, we evaluated in vivo the long-term effects of a single inoculation of Aβice or Aβ-wild-type (Aβwt) in the hippocampus of transgenic mice (APPswe/PS1dE9) over-expressing Aβ1-42 peptide. Interestingly, we found that the single intra-hippocampal inoculation of Aβice to mice rescued synaptic density and spatial memory losses four months post-inoculation, compared with Aβwt inoculation. Although Aβ load was not modulated by Aβice infusion, the amount of Tau-positive neuritic plaques was significantly reduced. Finally, a lower phagocytosis by microglia of post-synaptic compounds was detected in Aβice-inoculated animals, which can partly explain the increased density of synapses in the Aβice animals. Thus, a single event as Aβice inoculation can improve the fate of AD-associated pathology and phenotype in mice several months after the event. These results open unexpected fields to develop innovative therapeutic strategies against AD.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Similar articles
-
Long term worsening of amyloid pathology, cerebral function, and cognition after a single inoculation of beta-amyloid seeds with Osaka mutation.Acta Neuropathol Commun. 2023 Apr 22;11(1):66. doi: 10.1186/s40478-023-01559-0. Acta Neuropathol Commun. 2023. PMID: 37087498 Free PMC article.
-
Soluble Aβ levels correlate with cognitive deficits in the 12-month-old APPswe/PS1dE9 mouse model of Alzheimer's disease.Behav Brain Res. 2011 Sep 23;222(2):342-50. doi: 10.1016/j.bbr.2011.03.072. Epub 2011 Apr 14. Behav Brain Res. 2011. PMID: 21513747
-
Corilagin improves cognitive impairment in APP/PS1 mice by reducing Aβ generation and enhancing synaptic plasticity.Eur J Pharmacol. 2024 Oct 15;981:176893. doi: 10.1016/j.ejphar.2024.176893. Epub 2024 Aug 10. Eur J Pharmacol. 2024. PMID: 39134295
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
-
Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?J Biol Chem. 2018 Oct 5;293(40):15419-15428. doi: 10.1074/jbc.R118.003999. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143530 Free PMC article. Review.
References
-
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

