The human ATAD5 has evolved unique structural elements to function exclusively as a PCNA unloader
- PMID: 38871854
- PMCID: PMC11563871
- DOI: 10.1038/s41594-024-01332-4
The human ATAD5 has evolved unique structural elements to function exclusively as a PCNA unloader
Abstract
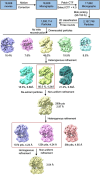
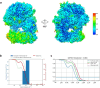
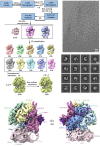
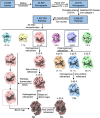
Humans have three different proliferating cell nuclear antigen (PCNA) clamp-loading complexes: RFC and CTF18-RFC load PCNA onto DNA, but ATAD5-RFC can only unload PCNA from DNA. The underlying structural basis of ATAD5-RFC unloading is unknown. We show here that ATAD5 has two unique locking loops that appear to tie the complex into a rigid structure, and together with a domain that plugs the DNA-binding chamber, prevent conformation changes required for DNA binding, likely explaining why ATAD5-RFC is exclusively a PCNA unloader. These features are conserved in the yeast PCNA unloader Elg1-RFC. We observe intermediates in which PCNA bound to ATAD5-RFC exists as a closed planar ring, a cracked spiral or a gapped spiral. Surprisingly, ATAD5-RFC can open a PCNA gap between PCNA protomers 2 and 3, different from the PCNA protomers 1 and 3 gap observed in all previously characterized clamp loaders.
© 2024. The Author(s).
Conflict of interest statement
Figures









Similar articles
-
The Atad5 RFC-like complex is the major unloader of proliferating cell nuclear antigen in Xenopus egg extracts.J Biol Chem. 2024 Jan;300(1):105588. doi: 10.1016/j.jbc.2023.105588. Epub 2023 Dec 21. J Biol Chem. 2024. PMID: 38141767 Free PMC article.
-
Regulation of PCNA cycling on replicating DNA by RFC and RFC-like complexes.Nat Commun. 2019 Jun 3;10(1):2420. doi: 10.1038/s41467-019-10376-w. Nat Commun. 2019. PMID: 31160570 Free PMC article.
-
Cryo-EM reveals a nearly complete PCNA loading process and unique features of the human alternative clamp loader CTF18-RFC.Proc Natl Acad Sci U S A. 2024 Apr 30;121(18):e2319727121. doi: 10.1073/pnas.2319727121. Epub 2024 Apr 26. Proc Natl Acad Sci U S A. 2024. PMID: 38669181 Free PMC article.
-
Is PCNA unloading the central function of the Elg1/ATAD5 replication factor C-like complex?Cell Cycle. 2013 Aug 15;12(16):2570-9. doi: 10.4161/cc.25626. Epub 2013 Jul 10. Cell Cycle. 2013. PMID: 23907118 Free PMC article. Review.
-
The PCNA-RFC families of DNA clamps and clamp loaders.Prog Nucleic Acid Res Mol Biol. 2004;78:227-60. doi: 10.1016/S0079-6603(04)78006-X. Prog Nucleic Acid Res Mol Biol. 2004. PMID: 15210332 Review.
Cited by
-
PCNA is a Nucleotide Exchange Factor for the Clamp Loader ATPase Complex.bioRxiv [Preprint]. 2025 Jul 3:2025.07.02.662830. doi: 10.1101/2025.07.02.662830. bioRxiv. 2025. PMID: 40631102 Free PMC article. Preprint.
-
The explosive discovery of TNT in early mouse embryos.Nat Struct Mol Biol. 2024 Jun;31(6):852-855. doi: 10.1038/s41594-024-01304-8. Nat Struct Mol Biol. 2024. PMID: 38789683 No abstract available.
References
-
- Kuriyan, J. & O’Donnell, M. Sliding clamps of DNA polymerases. J. Mol. Biol.234, 915–925 (1993). - PubMed
-
- Moldovan, G.-L., Pfander, B. & Jentsch, S. PCNA, the maestro of the replication fork. Cell129, 665–679 (2007). - PubMed
-
- Joyce, C. M. & Steitz, T. A. Function and structure relationships in DNA polymerases. Annu. Rev. Biochem.63, 777–822 (1994). - PubMed
-
- Krishna, T. S., Kong, X. P., Gary, S., Burgers, P. M. & Kuriyan, J. Crystal structure of the eukaryotic DNA polymerase processivity factor PCNA. Cell79, 1233–1243 (1994). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

