The path to leptomeningeal metastasis
- PMID: 38871881
- PMCID: PMC11404355
- DOI: 10.1038/s41568-024-00700-y
The path to leptomeningeal metastasis
Abstract
The leptomeninges, the cerebrospinal-fluid-filled tissues surrounding the central nervous system, play host to various pathologies including infection, neuroinflammation and malignancy. Spread of systemic cancer into this space, termed leptomeningeal metastasis, occurs in 5-10% of patients with solid tumours and portends a bleak clinical prognosis. Previous, predominantly descriptive, clinical studies have provided few insights. Recent development of preclinical leptomeningeal metastasis models, alongside genomic, transcriptomic and proteomic sequencing efforts, has provided groundwork for mechanistic understanding and identification of long-needed therapeutic targets. Although previously understood as an anatomically isolated compartment, the leptomeninges are increasingly appreciated as a major conduit of communication between the systemic circulation and the central nervous system. Despite the unique nature of the leptomeningeal microenvironment, the general principles of metastasis hold true: cells metastasizing to the leptomeninges must gain access to the new environment, survive within the space and evade the immune system. The study of leptomeningeal metastasis has the potential to uncover novel site-specific metastatic principles and illuminate the physiology of the leptomeningeal space. In this Review, we provide a biology-focused overview of how metastatic cells reach the leptomeninges, thrive in this nutritionally sparse environment and evade the detection of the omnipresent immune system.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests
J.R. and A.B. are inventors on the provisional US patent applications 63/449,817 and 63/449,823 and international patent application PCT/US24/18343. A.B. holds an unpaid position on the scientific advisory board for Evren Scientific and is an inventor on the US patents 62/258,044, 10/413,522 and 63/052,139.
Figures


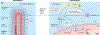
References
-
- Vanderah TW & Gould DJ Nolte’s the Human Brain : An Introduction to its Functional Anatomy 8th edn (Elsevier, 2021).
-
- Subira D et al. Diagnostic and prognostic significance of flow cytometry immunophenotyping in patients with leptomeningeal carcinomatosis. Clin. Exp. Metastasis 32, 383–391 (2015). - PubMed
-
- Magbanua MJ et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 73, 7134–7143 (2013). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

