Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: a phase 1 trial
- PMID: 38871975
- PMCID: PMC11405281
- DOI: 10.1038/s41591-024-03083-7
Botensilimab plus balstilimab in relapsed/refractory microsatellite stable metastatic colorectal cancer: a phase 1 trial
Abstract
Microsatellite stable metastatic colorectal cancer (MSS mCRC; mismatch repair proficient) has previously responded poorly to immune checkpoint blockade. Botensilimab (BOT) is an Fc-enhanced multifunctional anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibody designed to expand therapy to cold/poorly immunogenic solid tumors, such as MSS mCRC. BOT with or without balstilimab (BAL; anti-PD-1 antibody) is being evaluated in an ongoing expanded phase 1 study. The primary endpoint is safety and tolerability, which was evaluated separately in the dose-escalation portion of the study and in patients with MSS mCRC (using combined dose-escalation/dose-expansion data). Secondary endpoints include investigator-assessed RECIST version 1.1-confirmed objective response rate (ORR), disease control rate (DCR), duration of response (DOR) and progression-free survival (PFS). Here we present outcomes in 148 heavily pre-treated patients with MSS mCRC (six from the dose-escalation cohort; 142 from the dose-expansion cohort) treated with BOT and BAL, 101 of whom were considered response evaluable with at least 6 months of follow-up. Treatment-related adverse events (TRAEs) occurred in 89% of patients with MSS mCRC (131/148), most commonly fatigue (35%, 52/148), diarrhea (32%, 47/148) and pyrexia (24%, 36/148), with no grade 5 TRAEs reported and a 12% discontinuation rate due to a TRAE (18/148; data fully mature). In the response-evaluable population (n = 101), ORR was 17% (17/101; 95% confidence interval (CI), 10-26%), and DCR was 61% (62/101; 95% CI, 51-71%). Median DOR was not reached (NR; 95% CI, 5.7 months-NR), and median PFS was 3.5 months (95% CI, 2.7-4.1 months), at a median follow-up of 10.3 months (range, 0.5-42.6 months; data continuing to mature). The combination of BOT plus BAL demonstrated a manageable safety profile with no new immune-mediated safety signals and encouraging clinical activity with durable responses. ClinicalTrials.gov identifier: NCT03860272 .
© 2024. The Author(s).
Conflict of interest statement
A.B.E.-K. has received payment or honoraria from and has served in a consulting or advisory role for Agenus. Inc., AstraZeneca/MedImmune, Bayer, Bristol Myers Squibb, Eisai, EMD Serono, Exelixis, Gilead Sciences, Merck, QED Therapeutics, Qurient, Roche/Genentech, Senti Biosciences, Servier Laboratories and Tallac Therapeutics and received research funding from Astex Pharmaceuticals, AstraZeneca and Fulgent Genetics. N.H.S. has received consulting fees from ABL Bio, Agenus, Inc., AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis, Numab, Puretech, Revitope and Roche/Genentech; support for attending meetings and/or travel from AstraZeneca and Regeneron; and research funding from Agenus, Inc., AstraZeneca, Bristol Myers Squibb, Immunocore, Merck, Pfizer, Puretech, Regeneron and Roche/Genentech. B.B. has received research funding from Agenus, Inc. and NanoView Biosciences; received consulting fees from BioLineRx; received support for attending meetings and/or travel from Agenus, Inc. and Erytech Pharma; and has participated in a data safety monitoring board or advisory board for Blueprint Medicines and Enlivex Therapeutics. B.A.W. has served in a consulting or advisory role for Aadi Bioscience, Adcendo, Boehringer Ingelheim, Deciphera, Epizyme, Polaris and SpringWorks; received research funding from Exelixis; and received travel, accommodations or expenses support from Agenus, Inc. A.J.B. has received research funding from Agenus, Inc., AstraZeneca, Geistlich Pharma, Oncomatryx Biopharma and Panavance Therapeutics; received travel support from Agenus, Inc.; and received consulting or advisory fees from Exelixis, Merck and Oncolytics. G.K.A.-A. has received grants or contracts from Agenus, Inc., Arcus, AstraZeneca, BioNTech, Bristol Myers Squibb, Elicio Therapeutics, Genentech/Roche, Helsinn, the Parker Institute for Cancer Immunotherapy, Pertzye, Puma Biotechnology, QED Therapeutics and Yiviva; received consulting fees from Astellas, AstraZeneca, Autem, Berry Genomics, BioNTech, Boehringer Ingelheim, Bristol Myers Squibb, Eisai, Exelixis, FibrioGen, Genentech/Roche, Incyte, Ipsen, Merck, Merus, Neogene Therapeutics, Novartis, Servier Pharmaceuticals, Tempus, Thetis Pharmaceuticals, Vector Pharma and Yiviva; and received support for attending meetings and/or travel from Astellas, AstraZeneca, Autem, Berry Genomics, BioNTech, Bristol Myers Squibb, Boehringer Ingelheim, Eisai, Exelixis, Fibriogen, Genentech/Roche, Incyte, Ipsen, Merck, Merus, Neogene, Novartis, Servier, Tempus, Thetis, Vector and Yiviva. M.G.F. has received consulting fees from AstraZeneca, Bayer, Bristol Myers Squibb, Incyte, Mirati, Pfizer and Taiho and participated in a data safety monitoring or advisory board for Bayer, Eisai, Entos, Janssen, Merck, Mirati Therapeutics, Nouscom, Seattle Genetics and Xenthera. D.M. has received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from Guardant Health. J.C.M. has received research funding from Agenus, Inc.; received grants or contracts from Agenus, Inc., Alpine Immune Sciences, Amgen, BioEclipse Therapeutics, Fate Therapeutics, FujiFilm, Genentech, Ideaya Biosciences, ImmuneSensor Therapeutics, Iovance, Istari Oncology, Nektar Therapeutics, NovoCure, Repertoire Immune Medicines, Rubius, Senwha, Simcha, Storm Therapeutics, Synthorx, T-Scan, Trishula Therapeutics, the University of Arizona, Werewolf Therapeutics and Y-mAbs Therapeutics; received consulting fees from Adagene, Amunix Pharmaceuticals, Bristol Myers Squibb, Boxer Capital, Genome Insight, Incyte, Imaging Endpoints, IQVIA, Oberland Capital, Novotech CRO, Red Arrow Therapeutics and Thirona Bio; served in a leadership or fiduciary role for Caris Life Sciences; and received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from Caris Life Sciences, Daiichi Sankyo and TGen. S.S. has received payment or honoraria from Array BioPharma; served in a consulting or advisory role for Barricade Therapeutics, Celularity, Dracen Pharmaceuticals and Elevar Therapeutics; received research funding from Aadi Bioscience, Amal Therapeutics, Celgene, Dracen Pharmaceuticals, HonorHealth, Inhibrx, Merck, Nektar Therapuetics, Novartis, Plexxikon, Sirnaomics, Syndax Pharmaceuticals, Takeda, Tesaro, Toray Industries and Zai Lab; and holds stock and other ownership interests with Barricade Therapeutics, Beta Cat Pharmaceuticals, ConverGene, Elevar Therapeutics, HLB, Salarius Pharmaceuticals and Stingray Therapeutics. R.E.S. has received research funding from AstraZeneca and Merck; received consulting fees from Amgen, AstraZeneca, Daiichi Sankyo, GE HealthCare, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Janssen, Regeneron and Sanofi Aventis; and received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from the Binay Foundation, EMD Serono, GameOn!, Illumina, OncLive and Targeted Oncology. J.S. has received consulting fees from Agenus, Inc.; served on advisory boards for Alveo Technologies, APIM Therapeutics, Benevolent AI, Bryologyx, Celltrion, Certis, Clinical ink, Eli Lilly, Equilibre Biopharmaceuticals, Graviton Bioscience Corporation, Greenmantle, Heat Biologics, IO Labs, Onconox, Pear Bio, Vaccitech, Volvox, vTv Therapeutics and Zephyr AI; and received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from Celltrion and Eli Lilly. Since 2024, J.S. is on the board of directors for Graviton Biosciences BV, Etira Therapeutics and Portage and has served in a leadership or fiduciary role for BB Healthcare Trust PLC and Xerion Healthcare. A.M.T. declares receipt of clinical trial research funding (to The University of Texas MD Anderson Cancer Center) from Agenus, Inc., Immatics, Novocure, OBI Pharma, the Parker Institute for Cancer Immunotherapy, Tachyon, Tempus and Tvardi; fees for consulting or advisory roles from Avstera Therapeutics, Bioeclipse, BrYet, Diaccurate, MacroGenics, NEX-I and VinceRx; and travel expenses from the American Society of Clinical Oncology, Cancer Care Crossroads, GenomeWeb Conference and Precision Medicine World Conference. M.S.G. has served in a consulting or advisory role for Cardinal Healthcare, Imaging Endpoints, IQVIA, Medtronics, Onquality, Pfizer, Qualigen, Springworks Tx and Viracta; is co-owner of Sphinx Health Solutions; and received institutional research support from Agenus, Inc., BeiGene, DynamiCure, Fore, Genentech, NiKang, Novartis, Nimbus, OncoResponse, Plexxicon, Pyxis Oncology, Redhill Bio, Riboscience, Roche, SQZ Biotech, Syndax and Veru. G.K.S. has served in a consulting or advisory role for Aadi Subsidiary, Astex, Boehringer Ingelheim, Concarlo Therapeutics, Epizyme, Ipsen, Kirilys Therapeutics, OnCusp, Puretech Health, Sellas Life Sciences and Shanghai Pharma and holds stock in Bionaut and GenCirq. H.-J.L. has received consulting fees from Janssen and Pfizer and received payment or honoraria for lectures, presentations, speaker’s bureaus, manuscript writing or educational events from AstraZeneca, Bristol Myers Squibb, Genentech, Merck, Mirati and Regeneron. S.J.O., J.E.G., J.M.P., W.W., D.C., G.M., C.D. and K.R. are current or former employees of Agenus, Inc. and may hold stock/stock options. T.J.C. is a consultant for Agenus, Inc., Gmelich Chair, and has received funding from Guyre funds. The other authors declare no competing interests (A.P., A.S.B. and B.L.S.).
Figures
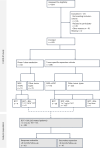

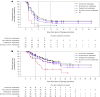
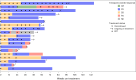

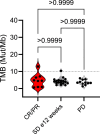
References
-
- American Cancer Society. Survival rates for colorectal cancer. https://www.cancer.org/cancer/types/colon-rectal-cancer/detection-diagno...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

