Fear learning induces synaptic potentiation between engram neurons in the rat lateral amygdala
- PMID: 38871992
- PMCID: PMC11239494
- DOI: 10.1038/s41593-024-01676-6
Fear learning induces synaptic potentiation between engram neurons in the rat lateral amygdala
Abstract
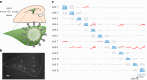
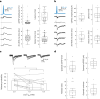
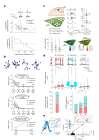
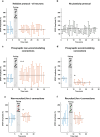
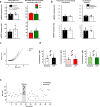
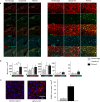
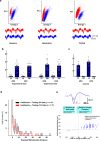
The lateral amygdala (LA) encodes fear memories by potentiating sensory inputs associated with threats and, in the process, recruits 10-30% of its neurons per fear memory engram. However, how the local network within the LA processes this information and whether it also plays a role in storing it are still largely unknown. Here, using ex vivo 12-patch-clamp and in vivo 32-electrode electrophysiological recordings in the LA of fear-conditioned rats, in combination with activity-dependent fluorescent and optogenetic tagging and recall, we identified a sparsely connected network between principal LA neurons that is organized in clusters. Fear conditioning specifically causes potentiation of synaptic connections between learning-recruited neurons. These findings of synaptic plasticity in an autoassociative excitatory network of the LA may suggest a basic principle through which a small number of pyramidal neurons could encode a large number of memories.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures










References
-
- Rogan MT, Stäubli UV, LeDoux JE. Fear conditioning induces associative long-term potentiation in the amygdala. Nature. 1997;390:604–607. - PubMed
-
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. - PubMed
-
- Han J-H, et al. Neuronal competition and selection during memory formation. Science. 2007;316:457–460. - PubMed
-
- Han J-H, et al. Selective erasure of a fear memory. Science. 2009;323:1492–1496. - PubMed
MeSH terms
Grants and funding
- IZLSZ3_148803/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- IZLSZ0_206045/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_192463/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- IZLSZ3_200297/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
- 310030_138526/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (Swiss National Science Foundation)
LinkOut - more resources
Full Text Sources

