A case of TAFRO syndrome after vaccination, successfully treated with cyclosporine
- PMID: 38872134
- PMCID: PMC11177486
- DOI: 10.1186/s12882-024-03630-x
A case of TAFRO syndrome after vaccination, successfully treated with cyclosporine
Abstract
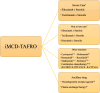
Background: TAFRO syndrome is a rare disorder that causes thrombocytopenia, generalized oedema, fever, organ enlargement, and renal impairment. Few reports have suggested an association with vaccines, and few cases have undergone renal biopsy. TAFRO syndrome is often severe and fatal, and its cause is unknown. We report a case of TAFRO syndrome that occurred after vaccination with the coronavirus disease 2019 (COVID-19) vaccine.
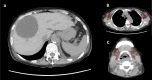
Case presentation: An 82-year-old woman received two doses of the BNT162b2 mRNA vaccine 3 weeks apart. Two weeks later, she was admitted to the hospital with oedema, accompanied with renal failure and thrombocytopenia. After close examination, she was diagnosed with TAFRO syndrome. She was treated with steroids, cyclosporine, and thrombopoietin receptor agonists. The patient was discharged after several months in remission.
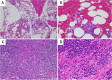
Conclusions: Although an incident of TAFRO syndrome after COVID-19 vaccination has been previously reported, this is a rare case in which the patient went into remission and was discharged. A renal biopsy was also performed in this case, which was consistent with previous reports. The favorable treatment course for TAFRO syndrome provides valuable insights.
Keywords: BNT162b2 mRNA; COVID-19; ITP (idiopathic thrombocytopenic purpura); MIS-A (multisystem inflammatory syndrome in adults); SCLS (systemic capillary leak syndrome); TAFRO syndrome.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Takai K, Nikkuni K, Shibuya H, Hashidate H. Thrombocytopenia with mild bone marrow fibrosis accompanied by fever, pleural effusion, ascites and hepatosplenomegaly. Rinsho Ketsueki. 2010;51:320–325. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

