A Cell Pre-Wrapping Seeding Technique for Hydrogel-Based Tubular Organ-On-A-Chip
- PMID: 38872259
- PMCID: PMC11321624
- DOI: 10.1002/advs.202400970
A Cell Pre-Wrapping Seeding Technique for Hydrogel-Based Tubular Organ-On-A-Chip
Abstract
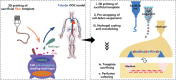
Organ-on-a-chip (OOC) models based on microfluidic technology are increasingly used to obtain mechanistic insight into (patho)physiological processes in humans, and they hold great promise for application in drug development and regenerative medicine. Despite significant progress in OOC development, several limitations of conventional microfluidic devices pose challenges. First, most microfluidic systems have rectangular cross sections and flat walls, and therefore tubular/ curved structures, like blood vessels and nephrons, are not well represented. Second, polymers used as base materials for microfluidic devices are much stiffer than in vivo extracellular matrix (ECM). Finally, in current cell seeding methods, challenges exist regarding precise control over cell seeding location, unreachable spaces due to flow resistances, and restricted dimensions/geometries. To address these limitations, an alternative cell seeding technique and a corresponding workflow is introduced to create circular cross-sectioned tubular OOC models by pre-wrapping cells around sacrificial fiber templates. As a proof of concept, a perfusable renal proximal tubule-on-a-chip is demonstrated with a diameter as small as 50 µm, cellular tubular structures with branches and curvature, and a preliminary vascular-renal tubule interaction model. The cell pre-wrapping seeding technique promises to enable the construction of diverse physiological/pathological models, providing tubular OOC systems for mechanistic investigations and drug development.
Keywords: cell pre‐wrapping seeding; hydrogel; renal proximal tubule; sacrificial template; tubular organ‐on‐a‐chip.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- a) Zanetti F., in Kidney‐on‐a‐chip." Organ‐on‐a‐chip Academic Press, 2020. 233.
- b) Kim S., LesherPerez S. C., Yamanishi C., Labuz J. M., Leung B., Takayama S., Biofabrication 2016, 8, 015021; - PubMed
- c) Sakolish C. M., Philip B., Mahler G. J., Biomicrofluidics 2019, 13, 014107; - PMC - PubMed
- d) Kim S., Takayama S., Kidney Res. Clin. Pract. 2015, 34, 165; - PMC - PubMed
- e) Ortiz A., Sanchez‐Nino M. D., Izquierdo M. C., Martin‐Cleary C., Garcia‐Bermejo L., Moreno J. A., Ruiz‐Ortega M., Draibe J., Cruzado J. M., Garcia‐Gonzalez M. A., Eur. J. Pharmacol. 2015, 759, 205. - PubMed
-
- a) Homan K. A., Kolesky D. B., Skylar‐Scott M. A., Herrmann J., Obuobi H., Moisan A., Lewis J. A., Sci. Rep. 2016, 6, 34845; - PMC - PubMed
- b) Ng C. P., Zhuang Y., Lin A. W. H., Teo J. C. M., Int. J. Tissue Eng. 2012, 2013, 319476;
- c) Wilmer M. J., Ng C. P., Lanz H. L., Vulto P., Suter‐Dick L., Masereeuw R., Trends Biotechnol. 2016, 34, 156; - PubMed
- d) Jang K.‐J., Mehr A. P., Hamilton G. A., McPartlin L. A., Chung S., Suh K.‐Y., Ingber D. E., Integr. Biol. 2013, 5, 1119. - PubMed
-
- a) Yi H.‐G., Lee H., Cho D.‐W., Bioengineering 2017, 4, 10; - PubMed
- b) Chen M. B., Srigunapalan S., Wheeler A. R., Simmons C. A., Lab Chip 2013, 13, 2591; - PubMed
- c) Coluccio M. L., Perozziello G., Malara N., Parrotta E., Zhang P., Gentile F., Limongi T., Raj P. M., Cuda G., Candeloro P., Microelectron. Eng. 2019, 208, 14;
- d) Sackmann E. K., Fulton A. L., Beebe D. J., Nature 2014, 507, 181; - PubMed
- e) Beebe D. J., Mensing G. A., Walker G. M., Annu. Rev. Biomed. Eng. 2002, 4, 261. - PubMed
-
- a) Weber E. J., Chapron A., Chapron B. D., Voellinger J. L., Lidberg K. A., Yeung C. K., Wang Z., Yamaura Y., Hailey D. W., Neumann T., Kidney Int. 2016, 90, 627; - PMC - PubMed
- b) Rodrigues R. O., Sousa P. C., Gaspar J., Bañobre‐López M., Lima R., Minas G., Small 2020, 16, 2003517; - PubMed
- c) Tomazelli Coltro W. K., Cheng C. M., Carrilho E., de Jesus D. P., Electrophoresis 2014, 35, 2309. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
