The TRIM28/miR133a/CD47 axis acts as a potential therapeutic target in pancreatic necrosis by impairing efferocytosis
- PMID: 38872307
- PMCID: PMC11403229
- DOI: 10.1016/j.ymthe.2024.06.005
The TRIM28/miR133a/CD47 axis acts as a potential therapeutic target in pancreatic necrosis by impairing efferocytosis
Abstract
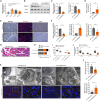
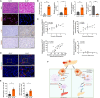
Efferocytosis, the clearance of apoptotic cells by macrophages, plays a crucial role in inflammatory responses and effectively prevents secondary necrosis. However, the mechanisms underlying efferocytosis in acute pancreatitis (AP) remain unclear. In this study, we demonstrated the presence of efferocytosis in injured human and mouse pancreatic tissues. We also observed significant upregulation of CD47, an efferocytosis-related the "do not eat me" molecule in injured acinar cells. Subsequently, we used CRISPR-Cas9 gene editing, anti-adeno-associated virus (AAV) gene modification, and anti-CD47 antibody to investigate the potential therapeutic role of AP. CD47 expression was negatively regulated by upstream miR133a, which is controlled by the transcription factor TRIM28. To further investigate the regulation of efferocytosis and reduction of pancreatic necrosis in AP, we used miR-133a-agomir and pancreas-specific AAV-shTRIM28 to modulate CD47 expression. Our findings confirmed that CD47-mediated efferocytosis is critical for preventing pancreatic necrosis and suggest that targeting the TRIM28-miR133a-CD47 axis is clinically relevant for the treatment of AP.
Keywords: acute pancreatitis; apoptotic cell clearance; clinical translation; immune microenvironment.
Copyright © 2024 The American Society of Gene and Cell Therapy. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Mederos M.A., Reber H.A., Girgis M.D. Acute Pancreatitis: A Review. Jama. 2021;325:382–390. - PubMed
-
- Sendler M., Dummer A., Weiss F.U., Krüger B., Wartmann T., Scharffetter-Kochanek K., van Rooijen N., Malla S.R., Aghdassi A., Halangk W., et al. Tumour necrosis factor α secretion induces protease activation and acinar cell necrosis in acute experimental pancreatitis in mice. Gut. 2013;62:430–439. - PubMed
-
- Sendler M., Weiss F.U., Golchert J., Homuth G., van den Brandt C., Mahajan U.M., Partecke L.I., Döring P., Gukovsky I., Gukovskaya A.S., et al. Cathepsin B-Mediated Activation of Trypsinogen in Endocytosing Macrophages Increases Severity of Pancreatitis in Mice. Gastroenterology. 2018;154:704–718.e10. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

