IRL790 modulated striatal D1 neurons synaptic plasticity ameliorating levodopa-induced dyskinesia in mouse
- PMID: 38872625
- PMCID: PMC11169859
- DOI: 10.3389/fnagi.2024.1401991
IRL790 modulated striatal D1 neurons synaptic plasticity ameliorating levodopa-induced dyskinesia in mouse
Abstract
Objective: Levodopa (L-dopa) therapy is the principal pharmacological treatment for Parkinson's disease (PD). Nevertheless, prolonged use of this drug may result in different involuntary movement symptoms caused by the medication, referred to as levodopa-induced dyskinesia (LID). LID is associated with changes in synaptic plasticity of the D1 medium spiny neurons (MSNs) located in the dorsal striatum (dStr). Within the striatum, the amount of Dopamine D3 receptor (D3R) is notably increased in LID, demonstrating colocalization with D1R expression in neurons, and the level of D3R expression is directly related to the intensity of LID. IRL 790, as a D3R antagonist, can ameliorate LID. This study aims to explore if IRL 790 improves LID by regulating the synaptic plasticity of D1+ MSNs in dStr.
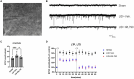
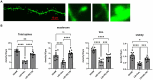
Methods: The electrophysiology and synaptic spine density of D1+ MSNs in dStr were recorded for sham mice, LID mice, and LID mice treated with IRL 790. The regulation of synaptic plasticity in LID D1+ MSNs by IRL 790 was analyzed. Behavioral tests were conducted to confirm the treatment effect of IRL 790 on LID.
Results: In LID D1+ MSNs, there was persistent abnormal LTP, absence of LTD, and an increase in spontaneous excitatory postsynaptic currents (sEPSCs). IRL 790 treatment restored normal LTP, LTD, and sEPSCs. Treatment with IRL 790 also restored the reduced dendritic spine density in D1+ MSNs of LID mice. IRL790 improved dyskinetic manifestations in LID mice.
Conclusion: IRL790 ameliorates LID by regulating the synaptic structure and functional plasticity of striatal D1+ MSNs.
Keywords: Parkinson’s disease; dopamine D3 receptor; functional plasticity; levodopa-induced dyskinesia; structural plasticity.
Copyright © 2024 Wang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Azkona G., Sagarduy A., Aristieta A., Vazquez N., Zubillaga V., Ruíz-Ortega J. A., et al. . (2014). Buspirone anti-dyskinetic effect is correlated with temporal normalization of dysregulated striatal DRD1 signalling in L-dopa-treated rats. Neuropharmacology 79, 726–737. doi: 10.1016/j.neuropharm.2013.11.024, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources

