Synergistic Effect of Transmyocardial Revascularization and Platelet-Rich Plasma on Improving Cardiac Function After Coronary Artery Bypass Grafting
- PMID: 38872704
- PMCID: PMC11170312
- DOI: 10.7759/cureus.60254
Synergistic Effect of Transmyocardial Revascularization and Platelet-Rich Plasma on Improving Cardiac Function After Coronary Artery Bypass Grafting
Abstract
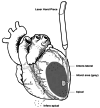
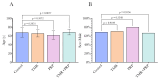
Background Coronary artery disease (CAD) is a global health burden, contributing to mortality and morbidity. A proportion of patients with CAD suffer from diffuse CAD, where conventional revascularization techniques such as percutaneous coronary intervention and coronary artery bypass grafting (CABG) may be insufficient to adequately restore myocardial perfusion. Transmyocardial revascularization (TMR) uses a laser to create microscopic channels in the myocardium, inducing inflammation, angiogenesis, and neovascularization to improve perfusion to ischemic regions. Platelet-rich plasma (PRP) is an autologous concentrate of platelets that contains a myriad of growth factors and bioactive proteins, which have been shown to promote tissue regeneration and wound healing. The combination of TMR and PRP therapies has been proposed to synergistically enhance myocardial revascularization and functional recovery in patients with advanced CAD undergoing surgical revascularization. Methods This study evaluated the efficacy of combining TMR and PRP with CABG in improving cardiac function in diffuse CAD patients. Fifty-two patients were randomized to CABG alone (n = 16), CABG+TMR (n = 17), CABG+PRP (n = 10), and CABG+TMR+PRP (n = 9). TMR was performed using a holmium:YAG laser to create 10 channels in the inferolateral left ventricular wall. PRP was prepared from autologous whole blood and injected into the myocardium adjacent to the TMR channels. Cardiac function was assessed using speckle-tracking echocardiography preoperatively, postoperatively, and at one-year follow-up. Adverse events, including post-operative atrial fibrillation, acute kidney injury, and readmissions, were also recorded. Statistical analyses were performed to compare outcomes between the treatment groups. Results The CABG+TMR+PRP group showed significantly improved global longitudinal strain (GLS) at one year compared to CABG alone (mean GLS -15.96 vs -12.09, p = 0.02). Post-operative left ventricular ejection fraction trended higher in the TMR+PRP group (57.78%) vs other groups, but not significantly. Post-operative atrial fibrillation was higher in the TMR+PRP group (67% vs 25%, p = 0.04), potentially reflecting increased inflammation. No significant differences were observed in other adverse events. Conclusions The results of this study suggest a synergistic benefit of combining TMR and PRP therapies as an adjunct to CABG in patients with diffuse CAD. The significant improvement in GLS at one year in the TMR+PRP group compared to CABG alone indicates enhanced myocardial remodeling and functional recovery, which may translate to improved long-term outcomes. The higher incidence of postoperative atrial fibrillation in the TMR+PRP group warrants further investigation but may reflect the heightened inflammatory response necessary for angiogenesis and tissue regeneration. Prospective, randomized controlled trials with larger sample sizes and longer follow-up periods are needed to validate these findings and optimize treatment protocols. Nonetheless, concomitant TMR+PRP therapy represents a promising approach to augmenting myocardial revascularization and recovery in patients with advanced CAD undergoing surgical revascularization.
Keywords: coronary artery bypass grafting (cabg); coronary artery disease (cad); platelet-rich plasma (prp); speckle tracking echocardiography (ste); transmyocardial revascularization (tmr).
Copyright © 2024, Khalpey et al.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
[Indications for transmyocardial laser therapy].Z Kardiol. 1996;85 Suppl 6:269-79. Z Kardiol. 1996. PMID: 9064976 Review. German.
-
[The effect of transmyocardial laser revascularization on anginal symptoms and clinical results in patients with incomplete surgical revascularization].Turk Kardiyol Dern Ars. 2009 Jun;37(4):246-52. Turk Kardiyol Dern Ars. 2009. PMID: 19717957 Turkish.
-
Long-Term Outcomes After CABG With Concomitant CO2 Transmyocardial Revascularization in Comparison With CABG Alone.Innovations (Phila). 2010 Mar;5(2):103-8. doi: 10.1097/IMI.0b013e3181d85935. Innovations (Phila). 2010. PMID: 22437356
-
Adjunctive transmyocardial revascularization: five-year follow-up of a prospective, randomized trial.Ann Thorac Surg. 2004 Aug;78(2):458-65; discussion 458-65. doi: 10.1016/j.athoracsur.2004.04.049. Ann Thorac Surg. 2004. PMID: 15276496 Clinical Trial.
-
Transmyocardial laser revascularization.J Card Surg. 2008 May-Jun;23(3):266-76. doi: 10.1111/j.1540-8191.2008.00579.x. J Card Surg. 2008. PMID: 18435649 Free PMC article. Review.
Cited by
-
Amniotic membrane, a novel bioscaffold in cardiac diseases: from mechanism to applications.Front Bioeng Biotechnol. 2024 Dec 20;12:1521462. doi: 10.3389/fbioe.2024.1521462. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39758951 Free PMC article. Review.
-
Angiogenesis in Atrial Fibrillation: A Literature Review.Biomedicines. 2025 Jun 6;13(6):1399. doi: 10.3390/biomedicines13061399. Biomedicines. 2025. PMID: 40564118 Free PMC article. Review.
-
Platelet-Rich Plasma in Cardiovascular Regeneration: Mechanistic Insights, Technological Innovations, and Future Directions.Rev Cardiovasc Med. 2025 Jul 28;26(7):39383. doi: 10.31083/RCM39383. eCollection 2025 Jul. Rev Cardiovasc Med. 2025. PMID: 40776966 Free PMC article. Review.
References
-
- Does the completeness of revascularization affect early survival after coronary artery bypass grafting in elderly patients? Osswald BR, Blackstone EH, Tochtermann U, Schweiger P, Thomas G, Vahl CF, Hagl S. Eur J Cardiothorac Surg. 2001;20:120-5, discussion 125-6. - PubMed
-
- Recurrence of angina after coronary artery bypass surgery: predictors and prognosis (CASS Registry). Coronary artery surgery study. Cameron AA, Davis KB, Rogers WJ. J Am Coll Cardiol. 1995;26:895–899. - PubMed
-
- Recurrent angina after coronary revascularization: a clinical challenge. Abbate A, Biondi-Zoccai GG, Agostoni P, Lipinski MJ, Vetrovec GW. Eur Heart J. 2007;28:1057–1065. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
