Identifying MAGE-A4-positive tumors for TCR T cell therapies in HLA-A∗02-eligible patients
- PMID: 38872830
- PMCID: PMC11170170
- DOI: 10.1016/j.omtm.2024.101265
Identifying MAGE-A4-positive tumors for TCR T cell therapies in HLA-A∗02-eligible patients
Abstract
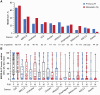
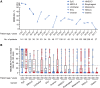
T cell receptor (TCR) T cell therapies target tumor antigens in a human leukocyte antigen (HLA)-restricted manner. Biomarker-defined therapies require validation of assays suitable for determination of patient eligibility. For clinical trials evaluating TCR T cell therapies targeting melanoma-associated antigen A4 (MAGE-A4), screening in studies NCT02636855 and NCT04044768 assesses patient eligibility based on: (1) high-resolution HLA typing and (2) tumor MAGE-A4 testing via an immunohistochemical assay in HLA-eligible patients. The HLA/MAGE-A4 assays validation, biomarker data, and their relationship to covariates (demographics, cancer type, histopathology, tissue location) are reported here. HLA-A∗02 eligibility was 44.8% (2,959/6,606) in patients from 43 sites across North America and Europe. While HLA-A∗02:01 was the most frequent HLA-A∗02 allele, others (A∗02:02, A∗02:03, A∗02:06) considerably increased HLA eligibility in Hispanic, Black, and Asian populations. Overall, MAGE-A4 prevalence based on clinical trial enrollment was 26% (447/1,750) across 10 solid tumor types, and was highest in synovial sarcoma (70%) and lowest in gastric cancer (9%). The covariates were generally not associated with MAGE-A4 expression, except for patient age in ovarian cancer and histology in non-small cell lung cancer. This report shows the eligibility rate from biomarker screening for TCR T cell therapies and provides epidemiological data for future clinical development of MAGE-A4-targeted therapies.
Keywords: HLA; MAGE-A4; T cell receptor; T cell therapy; assay validation; biomarker; clinical trial; immunotherapy; patient screening; precision medicine.
© 2024 The Authors.
Conflict of interest statement
T.W., J.-M.N., S.R., C.K., M.C., R.B., K.H., A.L., A.C., R.W., L.Q., J.P.S., C.M., F.B., E.E., P.B., and D.W. are or were employees of Adaptimmune at the time of the study and may own stock/stock options in Adaptimmune. M.V.K., S.V.R., K.S., and K.A. are employees of CellCarta NV, Antwerp, Belgium, and were involved in the development and validation of the MAGE-A4 IHC assay. G.B.J. received consulting fees from AbbVie, Daiichi Sankyo, Eli Lilly, Genzyme, Gilead, Merck Sharp & Dohme, Novartis, Regeneron; expenses from Regeneron; research funding from AstraZeneca, BeiGene, Bristol Myers Squibb, Daiichi Sankyo, Exelixis, Genentech, Incyte, Merck Sharp & Dohme, Novartis, Regeneron. M.O.B. received grant support from Merck, Takara Bio; quality improvement support from Novartis; is on the advisory board of Adaptimmune, Bristol Myers Squibb, GlaxoSmithKline, IDEAYA, Instil Bio, Iovance, La Roche Possey, Medison, Merck, Novartis, Pfizer, Regeneron, Sanofi, Sun Pharma; oral presentations given for Bristol Myers Squibb, Merck, Novartis, Pfizer, Sanofi. J.M.C. received grant/research support from AbbVie, Adaptimmune, Array, AstraZeneca, Bayer, Bristol Myers Squibb, CBMG, Genentech, GlaxoSmithKline, Grid Therapeutics, Medpacto, Moderna, Spectrum; consultant for Amgen, Corbus, G1 Therapeutics, Novartis, Omega, Sanofi, Turning Point, Vivacitas; speaker’s bureau for AstraZeneca. J.F.G. was compensated consultant or received honoraria from AI Proteins, AstraZeneca, Blueprint Medicines, Bristol Myers Squibb, Curie Therapeutics, Genentech/Roche, Gilead, iTeos, Jounce, Karyopharm, Loxo/Lilly, Merck, Merus Pharmacueticals, Mirati, Moderna, Novartis, Novocure, Nuvalent, Pfizer, Silverback Therapeutics, Takeda; research support from Genentech/Roche, Novartis, Takeda; institutional research support from Adaptimmune, Alexo, Array Biopharma, Bristol Myers Squibb, Blueprint Medicines, Jounce, Merck, Moderna, Novartis, Palleon, Tesaro; equity in AI Proteins; and has an immediate family member who is an employee with equity at Ironwood Pharmaceuticals. V.M. received consulting fees from Affimed, AstraZeneca, Bayer, Bristol Myers Squibb, Janssen, Roche, Syneos; was principal investigator – institutional funding from AbbVie, AceaBio, Adaptimmune, ADC Therapeutics, Aduro, Agenus, Amcure, Amgen, Astellas, AstraZeneca, Bayer, BeiGene, BioInvent International AB, Bristol Myers Squibb, Boehringer, Boston, Celgene, Daichii Sankyo, DEBIOPHARM, Eisai, e-Terapeutics, Exelisis, Forma Therapeutics, Genmab, GlaxoSmithKline, Harpoon, Hutchison, Immutep, Incyte, Inovio, Iovance, Janssen, Kyowa Kirin, Lilly, Loxo, MedSir, Menarini, Merck, Merus, Millennium, MSD, Nanobiotix, Nektar, Novartis, Odonate Therapeutics, Pfizer, Pharma Mar, PharmaMar, Principia, PsiOxus, Puma, Regeneron, Rigontec, Roche, Sanofi, Sierra Oncology, Synthon, Taiho, Takeda, Tesaro, Transgene, Turning Point Therapeutics, Upshersmith. M.J. received research funding (institutional) from AbbVie, Acerta, Adaptimmune, Amgen, Apexigen, Arcus Biosciences, Array BioPharma, Artios Pharma, AstraZeneca, Atreca, BeiGene, BerGenBio, BioAtla, Black Diamond, Boehringer Ingelheim, Bristol Myers Squibb, Calithera Biosciences, Carisma Therapeutics, Checkpoint Therapeutics, City of Hope National Medical Center, Corvus Pharmaceuticals, Curis, CytomX, Daiichi Sankyo, Dracen Pharmaceuticals, Dynavax, Eli Lilly, Elicio Therapeutics, EMD Serono, EQRx, Erasca, Exelixis, Fate Therapeutics, Genentech/Roche, Genmab, Genocea Biosciences, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Harpoon, Helsinn Healthcare SA, Hengrui Therapeutics, Hutchison MediPharma, IDEAYA Biosciences, IGM Biosciences, Immunitas Therapeutics, Immunocore, Incyte, Janssen, Jounce Therapeutics, Kadmon Pharmaceuticals, Kartos Therapeutics, Loxo Oncology, Lycera, Memorial Sloan Kettering, Merck, Merus, Mirati Therapeutics, Mythic Therapeutics, NeoImmune Tech, Neovia Oncology, Novartis, Numab Therapeutics, Nuvalent, OncoMed Pharmaceuticals, Palleon Pharmaceuticals, Pfizer, PMV Pharmaceuticals, Rain Therapeutics, RasCal Therapeutics, Regeneron Pharmaceuticals, Relay Therapeutics, Revolution Medicines, Ribon Therapeutics, Rubius Therapeutics, Sanofi, Seven and Eight Biopharmaceuticals/Birdie Biopharmaceuticals, Shattuck Labs, Silicon Therapeutics, Stem CentRx, Syndax Pharmaceuticals, Takeda Pharmaceuticals, Tarveda, TCR2 Therapeutics, Tempest Therapeutics, Tizona Therapeutics, TMUNITY Therapeutics, Turning Point Therapeutics, University of Michigan, Vyriad, WindMIL Therapeutics, Y-mAbs Therapeutics; had a consulting/advisory role (institutional) for AbbVie, Amgen, Arcus Biosciences, Arrivent, Astellas, AstraZeneca, Black Diamond, Boehringer Ingelheim, Calithera Biosciences, Daiichi Sankyo, EcoR1, Genentech/Roche, Genmab, Genocea Biosciences, Gilead Sciences, GlaxoSmithKline, Gritstone Oncology, Ideaya Biosciences, Immunocore, iTeos, Janssen, Jazz Pharmaceuticals, Merck, Mirati Therapeutics, Molecular Axiom, Normunity, Novartis, Oncorus, Pyramid Biosciences, Regeneron Pharmaceuticals, Revolution Medicines, Sanofi-Aventis, SeaGen, Synthekine, Takeda Pharmaceuticals, Turning Point Therapeutics, VBL Therapeutics. D.S.H. received travel, accommodations, expenses from AACR, ASCO, CLCC, Bayer, Genmab, SITC, Telperian; had consulting, speaker, or advisory role with 28Bio, AbbVie, Acuta, Adaptimmune, Alkermes, Alpha Insights, Amgen, Affini-T, Astellas, Aumbiosciences, Axiom, Baxter, Bayer, Boxer Capital, BridgeBio, CARSgen, CLCC, COG, COR2ed, Cowen, EcoR1, Erasca, Fate Therapeutics, F. Hoffmann-La Roche, Genentech, Gennao Bio, Gilead, GLG, Group H, Guidepoint, HCW Precision Oncology, Immunogenesis, InduPro, Janssen, Liberium, MedaCorp, Medscape, Numab, Oncologia Brasil, ORI Capital, Pfizer, Pharma Intelligence, POET Congress, Prime Oncology, Projects in Knowledge, Quanta, RAIN, Ridgeline, SeaGen, Stanford, STCube, Takeda, Tavistock, Trieza Therapeutics, Turning Point Therapeutics, WebMD, YingLing Pharma, Ziopharm; has other ownership interests in Molecular Match (advisor), OncoResponse (founder, advisor), Telperian (founder, advisor); received institutional research/grant funding from AbbVie, Adaptimmune, Adlai-Nortye, Amgen, AstraZeneca, Bayer, Biomea, Bristol Myers Squibb, Daiichi Sankyo, Deciphera, Eisai, Eli Lilly, Endeavor, Erasca, F. Hoffmann-La Roche, Fate Therapeutics, Genentech, Genmab, Immunogenesis, Infinity, Kyowa Kirin, Merck, Mirati, Navier, NCI-CTEP, Novartis, Numab, Pfizer, Pyramid Bio, Revolution Medicine, SeaGen, STCube, Takeda, TCR2, Turning Point Therapeutics, VM Oncology.
Figures




References
-
- Creelan B.C., Wang C., Teer J.K., Toloza E.M., Yao J., Kim S., Landin A.M., Mullinax J.E., Saller J.J., Saltos A.N., et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat. Med. 2021;27:1410–1418. doi: 10.1038/s41591-021-01462-y. - DOI - PMC - PubMed
-
- Sarnaik A.A., Hamid O., Khushalani N.I., Lewis K.D., Medina T., Kluger H.M., Thomas S.S., Domingo-Musibay E., Pavlick A.C., Whitman E.D., et al. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma. J. Clin. Oncol. 2021;39:2656–2666. doi: 10.1200/JCO.21.00612. - DOI - PMC - PubMed
-
- Stevanovic S., Draper L.M., Langhan M.M., Campbell T.E., Kwong M.L., Wunderlich J.R., Dudley M.E., Yang J.C., Sherry R.M., Kammula U.S., et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. J. Clin. Oncol. 2015;33:1543–1550. doi: 10.1200/JCO.2014.58.9093. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

