Insulated π-conjugated 2,2'-bipyridine transition-metal complexes: enhanced photoproperties in luminescence and catalysis
- PMID: 38873064
- PMCID: PMC11168077
- DOI: 10.1039/d4sc01046a
Insulated π-conjugated 2,2'-bipyridine transition-metal complexes: enhanced photoproperties in luminescence and catalysis
Abstract
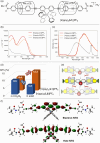
2,2'-Bipyridine has been identified as a privileged ligand scaffold for photofunctional transition metal complexes. We herein report on the synthesis and photoproperties of an insulated π-conjugated 2,2'-bipyridine with a linked rotaxane structure consisting of permethylated α-cyclodextrin (PM α-CD) and oligo(p-phenylene ethynylene). The insulated π-conjugated 2,2'-bipyridine exhibited enhanced ligand performance in the solid-state emitting biscyclometalated Ir complexes and visible-light-driven Ni catalysts owing to π-extension and remote steric effects based on the linked rotaxane structure.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Synthesis of an organic-soluble π-conjugated [3]rotaxane via rotation of glucopyranose units in permethylated β-cyclodextrin.Beilstein J Org Chem. 2014 Nov 28;10:2800-8. doi: 10.3762/bjoc.10.297. eCollection 2014. Beilstein J Org Chem. 2014. PMID: 25550746 Free PMC article.
-
A Typical Metal-Ion-Responsive Color-Tunable Emitting Insulated π-Conjugated Polymer Film.Angew Chem Int Ed Engl. 2016 Oct 17;55(43):13427-13431. doi: 10.1002/anie.201603160. Epub 2016 Jul 7. Angew Chem Int Ed Engl. 2016. PMID: 27383238
-
Programmed Synthesis of Molecular Wires with Fixed Insulation and Defined Length Based on Oligo(phenylene ethynylene) and Permethylated α-Cyclodextrins.Chemistry. 2017 Oct 26;23(60):15073-15079. doi: 10.1002/chem.201701428. Epub 2017 Jul 19. Chemistry. 2017. PMID: 28577322
-
π-Conjugated molecules covered by permethylated cyclodextrins.Chem Rec. 2011 Sep;11(5):269-83. doi: 10.1002/tcr.201100009. Epub 2011 Sep 7. Chem Rec. 2011. PMID: 21898775 Review.
-
Luminescent ionic transition-metal complexes for light-emitting electrochemical cells.Angew Chem Int Ed Engl. 2012 Aug 13;51(33):8178-211. doi: 10.1002/anie.201201471. Angew Chem Int Ed Engl. 2012. PMID: 22887710 Review.
Cited by
-
Investigation of Optical Properties and Biological Activities of Antimony(III) Halide Templated by 2,2'-Bipyridinium for Optoelectronic Devices.ACS Omega. 2025 Apr 29;10(18):19107-19114. doi: 10.1021/acsomega.5c01763. eCollection 2025 May 13. ACS Omega. 2025. PMID: 40385195 Free PMC article.
References
-
- Ward M. D. White C. M. Barigelletti F. Armaroli N. Calogero G. Flamigni L. Coord. Chem. Rev. 1998;171:481–488. doi: 10.1016/S0010-8545(98)90071-6. - DOI
-
- Munegowda M. A. Manalac A. Weersink M. McFarland S. A. Lilge L. Coord. Chem. Rev. 2022;470:214712. doi: 10.1016/j.ccr.2022.214712. - DOI - PMC - PubMed
- Teets T. S. and Wu Y., Organometallic Photosensitizers, In Comprehensive Organometallic Chemistry IV, ed. G. Parkin, K. Meyer and D. O'hare, Elsevier, Oxford, UK, 2022, pp. 284–338
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

