De-epimerizing DyKAT of β-lactones generated by isothiourea-catalysed enantioselective [2 + 2] cycloaddition
- PMID: 38873072
- PMCID: PMC11168096
- DOI: 10.1039/d4sc01410c
De-epimerizing DyKAT of β-lactones generated by isothiourea-catalysed enantioselective [2 + 2] cycloaddition
Abstract
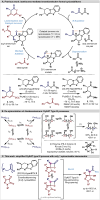
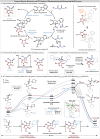
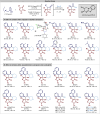
An enantioselective isothiourea-catalysed [2 + 2] cycloaddition of C(1)-ammonium enolates with pyrazol-4,5-diones is used to construct spirocyclic β-lactones in good yields, excellent enantioselectivity (99 : 1 er) but with modest diastereocontrol (typically 70 : 30 dr). Upon ring-opening with morpholine or alternative nucleophilic amines and alcohols β-hydroxyamide and β-hydroxyester products are generated with enhanced diastereocontrol (up to >95 : 5 dr). Control experiments show that stereoconvergence is observed in the ring-opening of diastereoisomeric β-lactones, leading to a single product (>95 : 5 dr, >99 : 1 er). Mechanistic studies and DFT analysis indicate a substrate controlled Dynamic Kinetic Asymmetric Transformation (DyKAT) involving epimerisation at C(3) of the β-lactone under the reaction conditions, coupled with a hydrogen bond-assisted nucleophilic addition to the Si-face of the β-lactone and stereodetermining ring-opening. The scope and limitations of a one-pot protocol consisting of isothiourea-catalysed enantio-determining [2 + 2] cycloaddition followed by diastereo-determining ring-opening are subsequently developed. Variation within the anhydride ammonium enolate precursor, as well as N(1) and C(3) within the pyrazol-4,5-dione scaffold is demonstrated, giving a range of functionalised β-hydroxyamides with high diastereo- and enantiocontrol (>20 examples, up to >95 : 5 dr and >99 : 1 er) via this DyKAT.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Albrecht Ł., Albrecht A. and Dell'Amico L., Asymmetric Organocatalysis: New Strategies, Catalysts, and Opportunities, Wiley-VCH GmbH, Weinheim, 1st edn, 2023
LinkOut - more resources
Full Text Sources
Miscellaneous

