Metal-free platforms for molecular thin films as high-performance supercapacitors
- PMID: 38873075
- PMCID: PMC11168099
- DOI: 10.1039/d4sc00611a
Metal-free platforms for molecular thin films as high-performance supercapacitors
Abstract
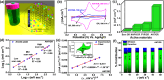
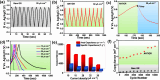
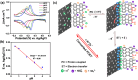
Controlling chemical functionalization and achieving stable electrode-molecule interfaces for high-performance electrochemical energy storage applications remain challenging tasks. Herein, we present a simple, controllable, scalable, and versatile electrochemical modification approach of graphite rods (GRs) extracted from low-cost Eveready cells that were covalently modified with anthracene oligomers. The anthracene oligomers with a total layer thickness of ∼24 nm on the GR electrode yield a remarkable specific capacitance of ∼670 F g-1 with good galvanostatic charge-discharge cycling stability (10 000) recorded in 1 M H2SO4 electrolyte. Such a boost in capacitance is attributed mainly to two contributions: (i) an electrical double-layer at the anthracene oligomer/GR/electrolyte interfaces, and (ii) the proton-coupled electron transfer (PCET) reaction, which ensures a substantial faradaic contribution to the total capacitance. Due to the higher conductivity of the anthracene films, it possesses more azo groups (-N[double bond, length as m-dash]N-) during the electrochemical growth of the oligomer films compared to pyrene and naphthalene oligomers, which is key to PCET reactions. AC-based electrical studies unravel the in-depth charge interfacial electrical behavior of anthracene-grafted electrodes. Asymmetrical solid-state supercapacitor devices were made using anthracene-modified biomass-derived porous carbon, which showed improved performance with a specific capacitance of ∼155 F g-1 at 2 A g-1 with an energy density of 5.8 W h kg-1 at a high-power density of 2010 W kg-1 and powered LED lighting for a longer period. The present work provides a promising metal-free approach in developing organic thin-film hybrid capacitors.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. RG, AM, and PCM have filed a provisional Indian patent application (Application No. 202211060927) lodged with the IIT Kanpur based on the work reported here.
Figures



References
-
- Yu M. Peng Y. Wang X. Ran F. Adv. Funct. Mater. 2023;33:2301877. doi: 10.1002/adfm.202301877. - DOI
-
- Salanne M. Rotenberg B. Naoi K. Kaneko K. Taberna P.-L. Grey C. P. Dunn B. Simon P. Nat. Energy. 2016;1:16070. doi: 10.1038/nenergy.2016.70. - DOI
-
- Yang P. Mai W. Nano Energy. 2014;8:274–290. doi: 10.1016/j.nanoen.2014.05.022. - DOI
-
- Rawlings D. Lee D. Kim J. Magdǎu I. B. Pace G. Richardson P. M. Thomas E. M. Danielsen S. P. O. Tolbert S. H. Miller T. F. Seshadri R. Segalman R. A. Chem. Mater. 2021;33:6464–6474. doi: 10.1021/acs.chemmater.1c01811. - DOI
LinkOut - more resources
Full Text Sources

