Electrochemical recycling of polymeric materials
- PMID: 38873080
- PMCID: PMC11168094
- DOI: 10.1039/d4sc01754d
Electrochemical recycling of polymeric materials
Abstract
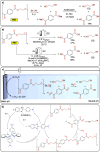
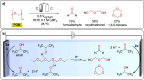
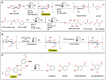
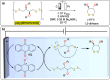
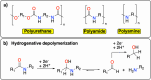
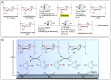
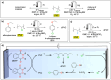
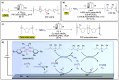
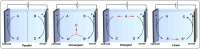
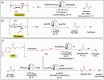
Polymeric materials play a pivotal role in our modern world, offering a diverse range of applications. However, they have been designed with end-properties in mind over recyclability, leading to a crisis in their waste management. The recent emergence of electrochemical recycling methodologies for polymeric materials provides new perspectives on closing their life cycle, and to a larger extent, the plastic loop by transforming plastic waste into monomers, building blocks, or new polymers. In this context, we summarize electrochemical strategies developed for the recovery of building blocks, the functionalization of polymer chains as well as paired electrolysis and discuss how they can make an impact on plastic recycling, especially compared to traditional thermochemical approaches. Additionally, we explore potential directions that could revolutionize research in electrochemical plastic recycling, addressing associated challenges.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Similar articles
-
The Minderoo-Monaco Commission on Plastics and Human Health.Ann Glob Health. 2023 Mar 21;89(1):23. doi: 10.5334/aogh.4056. eCollection 2023. Ann Glob Health. 2023. PMID: 36969097 Free PMC article. Review.
-
Polydiketoenamines for a Circular Plastics Economy.Acc Chem Res. 2022 Oct 4;55(19):2753-2765. doi: 10.1021/acs.accounts.2c00308. Epub 2022 Sep 15. Acc Chem Res. 2022. PMID: 36108255
-
Plastic waste from recycling centres: Characterisation and evaluation of plastic recyclability.Waste Manag. 2019 Jul 15;95:388-398. doi: 10.1016/j.wasman.2019.06.038. Epub 2019 Jun 25. Waste Manag. 2019. PMID: 31351625
-
Closing the loop for PET, PE and PP waste from households: Influence of material properties and product design for plastic recycling.Waste Manag. 2019 Aug 1;96:75-85. doi: 10.1016/j.wasman.2019.07.005. Epub 2019 Jul 16. Waste Manag. 2019. PMID: 31376972
-
Approaches for Management and Valorization of Non-Homogeneous, Non-Recyclable Plastic Waste.Int J Environ Res Public Health. 2022 Aug 15;19(16):10088. doi: 10.3390/ijerph191610088. Int J Environ Res Public Health. 2022. PMID: 36011719 Free PMC article. Review.
Cited by
-
Recent Advances in the Use of Ionic Liquids and Deep Eutectic Solvents for Lignocellulosic Biorefineries and Biobased Chemical and Material Production.Chem Rev. 2025 Jun 25;125(12):5461-5583. doi: 10.1021/acs.chemrev.4c00754. Epub 2025 Jun 6. Chem Rev. 2025. PMID: 40479538 Free PMC article. Review.
-
Harnessing Non-Thermal External Stimuli for Polymer Recycling.Macromolecules. 2025 Feb 18;58(5):2210-2223. doi: 10.1021/acs.macromol.4c02690. eCollection 2025 Mar 11. Macromolecules. 2025. PMID: 40104264 Free PMC article. Review.
-
Electrochemical Commodity Polymer Up- and Re-Cycling: Toward Sustainable and Circular Plastic Treatment.Macromol Rapid Commun. 2025 Aug;46(15):e2500143. doi: 10.1002/marc.202500143. Epub 2025 Apr 18. Macromol Rapid Commun. 2025. PMID: 40249382 Free PMC article.
References
-
- Plastic waste and recycling in the EU, https://www.europarl.europa.eu/news/en/headlines/society/20181212STO2161..., accessed September 26, 2023
-
- Singh N. Hui D. Singh R. Ahuja I. P. S. Feo L. Fraternali F. Composites, Part B. 2017;115:409–422. doi: 10.1016/j.compositesb.2016.09.013. - DOI
-
- Rahimi A. García J. M. Nat. Rev. Chem. 2017;1:1–11. doi: 10.1038/s41570-016-0001. - DOI
Publication types
LinkOut - more resources
Full Text Sources

