An overview of magnesium-based implants in orthopaedics and a prospect of its application in spine fusion
- PMID: 38873086
- PMCID: PMC11170442
- DOI: 10.1016/j.bioactmat.2024.04.026
An overview of magnesium-based implants in orthopaedics and a prospect of its application in spine fusion
Abstract
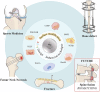
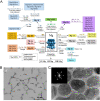
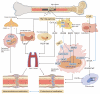
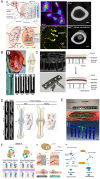
Due to matching biomechanical properties and significant biological activity, Mg-based implants present great potential in orthopedic applications. In recent years, the biocompatibility and therapeutic effect of magnesium-based implants have been widely investigated in trauma repair. In contrast, the R&D work of Mg-based implants in spinal fusion is still limited. This review firstly introduced the general background for Mg-based implants. Secondly, the mechanical properties and degradation behaviors of Mg and its traditional and novel alloys were reviewed. Then, different surface modification techniques of Mg-based implants were described. Thirdly, this review comprehensively summarized the biological pathways of Mg degradation to promote bone formation in neuro-musculoskeletal circuit, angiogenesis with H-type vessel formation, osteogenesis with osteoblasts activation and chondrocyte ossification as an integrated system. Fourthly, this review followed the translation process of Mg-based implants via updating the preclinical studies in fracture fixation, sports trauma repair and reconstruction, and bone distraction for large bone defect. Furthermore, the pilot clinical studies were involved to demonstrate the reliable clinical safety and satisfactory bioactive effects of Mg-based implants in bone formation. Finally, this review introduced the background of spine fusion surgeryand the challenges of biological matching cage development. At last, this review prospected the translation potential of a hybrid Mg-PEEK spine fusion cage design.
Keywords: Magnesium; Magnesium alloys; Magnesium implants; Magnesium surface modification; Spine fusion.
© 2024 The Authors.
Conflict of interest statement
This study was supported by 10.13039/501100009592Beijing Municipal Science and Technology Project (Z201100005520073), Key Clinical projects of Peking University Third hospital (BYSY2022064), 10.13039/501100002858China Postdoctoral Science Foundation (M2023740146), and 10.13039/501100001809National Natural Science Foundation of China (82302731). Yufeng Zheng is editor-in-chief for Bioactive Materials and was not involved in the editorial review or the decision to publish this article. All authors declare that there are no competing interests.
Figures







References
-
- Manam N., Harun W., Shri D., Ghani S., Kurniawan T., Ismail M.H., Ibrahim M. Study of corrosion in biocompatible metals for implants: a review. J. Alloys Compd. 2017;701:698–715.
-
- Williams B.R., McCreary D.L., Chau M., Cunningham B.P., Pena F., Swiontkowski M.F. Functional outcomes of symptomatic implant removal following ankle fracture open reduction and internal fixation. Foot Ankle Int. 2018;39(6):674–680. - PubMed
-
- Lovald S., Mercer D., Hanson J., Cowgill I., Erdman M., Robinson P., Diamond B. Complications and hardware removal after open reduction and internal fixation of humeral fractures. J. Trauma. 2011;70(5):1273–1277. ; discussion 1277-8. - PubMed
-
- Ostergaard P.J., Hall M.J., Xiong G., Zhang D., Earp B.E. Risk factors for implant removal after surgical fixation of midshaft clavicle fractures. Orthopedics. 2022;45(4):e201–e206. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

