Production and characterization of rhamnolipid biosurfactant from thermophilic Geobacillus stearothermophilus bacterium isolated from Uhud mountain
- PMID: 38873141
- PMCID: PMC11173098
- DOI: 10.3389/fmicb.2024.1358175
Production and characterization of rhamnolipid biosurfactant from thermophilic Geobacillus stearothermophilus bacterium isolated from Uhud mountain
Abstract
Introduction: Biosurfactants have been given considerable attention as they are potential candidates for several biotechnological applications.
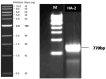
Materials and methods: In this study, a promising thermophilic biosurfactant-producing HA-2 was isolated from the volcanic and arid region of Uhud mountain, Madinah, Saudi Arabia. It was identified using 16S rRNA gene sequence analysis. The biosurfactant production ability was screened using different methods such as the drop collapse test, oil spreading test, hemolytic activity test, CTAB test, and emulsification index. The ability of rhamnolipid production by the tested strain was confirmed by the polymerase chain reaction (PCR) of rhlAB. The affinity of thermophilic HA-2 to hydrophobic substrates was also investigated. Optimization of biosurfactant production was conducted. The biological activities of produced surfactant were investigated.
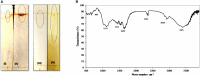
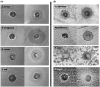
Results and discussion: The isolated HA-1 was identified as Geobacillus stearothermophilus strain OR911984. It could utilize waste sunflower frying oil (WSFF) oil as a low-cost carbon source. It showed high emulsification activity (52 ± 0.0%) and positive results toward other biosurfactant screening tests. The strain showed high cell adhesion to hexane with 41.2% cell surface hydrophobicity. Fourier-transform infrared (FTIR) spectra indicated the presence of hydrophobic chains that comprise lipids, sugars, and hydrophilic glycolipid components. The optimization results showed the optimal factors included potato peel as a carbon source with 68.8% emulsification activity, yeast extract as a nitrogen source with 60% emulsification activity, a pH of 9 (56.6%), and a temperature of 50° (72%). The kinetics showed that optimum biosurfactant production (572.4 mg/L) was recorded at 5 days of incubation. The produced rhamnolipid biosurfactant showed high antimicrobial activity against some human and plant pathogenic bacterial and fungal isolates and high antioxidant activity (90.4%). In addition, it enhanced wheat (Triticum aestivum) growth, with the greatest enhancement obtained with the 5% concentration. Therefore, thermophilic G. stearothermophilus is a promising rhamnolipid biosurfactant producer that utilizes many organic wastes. The produced biosurfactant could be applied as a promising emulsifier, antimicrobial, antioxidant, and plant growth promoter.
Keywords: Geobacillus; antimicrobial activity; biosurfactants; rhamnolipid; rhl gene; thermophilic bacteria.
Copyright © 2024 Albasri, Almohammadi, Alhhazmi, Bukhari, Waznah and Mawad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Polyphasic Analysis Reveals Potential Petroleum Hydrocarbon Degradation and Biosurfactant Production by Rare Biosphere Thermophilic Bacteria From Deception Island, an Active Antarctic Volcano.Front Microbiol. 2022 May 4;13:885557. doi: 10.3389/fmicb.2022.885557. eCollection 2022. Front Microbiol. 2022. PMID: 35602031 Free PMC article.
-
Production and characterization of rhamnolipid biosurfactant from waste frying coconut oil using a novel Pseudomonas aeruginosa D.J Appl Microbiol. 2013 Feb;114(2):373-83. doi: 10.1111/jam.12069. Epub 2013 Jan 7. J Appl Microbiol. 2013. PMID: 23164038
-
Exploration and characterization of a newly isolated bacterium, Enterobacter quasihormaechei strain BDIFST24001, capable of producing rhamnolipid biosurfactant for oil remediation.Access Microbiol. 2024 Aug 1;6(8):000830.v4. doi: 10.1099/acmi.0.000830.v4. eCollection 2024. Access Microbiol. 2024. PMID: 39100884 Free PMC article.
-
Characterization and emulsification properties of rhamnolipid and sophorolipid biosurfactants and their applications.Int J Mol Sci. 2011 Feb 18;12(2):1232-44. doi: 10.3390/ijms12021232. Int J Mol Sci. 2011. PMID: 21541055 Free PMC article. Review.
-
Critical review on biosurfactant analysis, purification and characterization using rhamnolipid as a model biosurfactant.Bioresour Technol. 2017 May;232:389-397. doi: 10.1016/j.biortech.2017.02.047. Epub 2017 Feb 16. Bioresour Technol. 2017. PMID: 28238638 Review.
Cited by
-
Sustainable Recovery of Critical Minerals from Wastes by Green Biosurfactants: A Review.Molecules. 2025 Jun 4;30(11):2461. doi: 10.3390/molecules30112461. Molecules. 2025. PMID: 40509347 Free PMC article. Review.
-
Rhamnolipids bio-production and miscellaneous applications towards green technologies: a literature review.PeerJ. 2025 Feb 21;13:e18981. doi: 10.7717/peerj.18981. eCollection 2025. PeerJ. 2025. PMID: 40247838 Free PMC article. Review.
-
Characterization of a New Glycolipopeptide Biosurfactant Produced by a Chrysene-Degrading Strain Achromobacter aegrifaciens.Appl Biochem Biotechnol. 2025 Aug;197(8):5200-5220. doi: 10.1007/s12010-025-05247-8. Epub 2025 May 26. Appl Biochem Biotechnol. 2025. PMID: 40418312
-
Metagenomic Analysis and Core Flooding Reveals the Indigenous Bacterial Community Information and MEOR Potential of the Main Water-Drive Low-Permeability Reservoir in the Ordos Basin.ACS Omega. 2025 May 28;10(22):23272-23280. doi: 10.1021/acsomega.5c01671. eCollection 2025 Jun 10. ACS Omega. 2025. PMID: 40521483 Free PMC article.
-
Investigation into the Enhancement Effects of Combined Bioremediation of Petroleum-Contaminated Soil Utilizing Immobilized Microbial Consortium and Sudan Grass.Toxics. 2025 Jul 16;13(7):599. doi: 10.3390/toxics13070599. Toxics. 2025. PMID: 40711043 Free PMC article.
References
-
- Abalos A., Pinazo A., Infante M., Casals M., Garcia F., Manresa A. (2001). Physicochemical and antimicrobial properties of new rhamnolipids produced by Pseudomonas aeruginosa AT10 from soybean oil refinery wastes. Langmuir 17, 1367–1371. 10.1021/la0011735 - DOI
-
- Abbasi H., Hamedi M. M., Lotfabad T. B., Zahiri H. S., Sharafi H., Masoomi F., et al. . (2012). Biosurfactant-producing bacterium, Pseudomonas aeruginosa MA01 isolated from spoiled apples: physicochemical and structural characteristics of isolated biosurfactant. J. Biosci. Bioeng. 113, 211–219. 10.1016/j.jbiosc.2011.10.002 - DOI - PubMed
-
- Adetunji C. O., Olaniyan O. T., Anani O. A., Inobeme A., Samson A. O., Oloke J. K., et al. . (2022). “Role of biosurfactant in the destruction of pores and destabilization of the biological membrane of pathogenic microorganisms,” in Green Sustainable Process for Chemical and Environmental Engineering and Science, eds. Inamuddin, C. O. Adetunji, and M. I. Ahame (Elsevier), 175–188.
-
- Aguirre-Noyola J. L., Ramírez Y. R., Ledezma J. R., Forero A. F., Rodríguez R. L., Jiménez J. T. (2020). Biosurfactants produced by metal-resistant Pseudomonas aeruginosa isolated from Zea mays rhizosphere and compost. Revista de Investigación Agraria y Ambiental 12, 101–112. 10.22490/21456453.3849 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

