Development of a new hazard scoring system in primary neuronal cell cultures for drug-induced acute neuronal toxicity identification in early drug discovery
- PMID: 38873414
- PMCID: PMC11170107
- DOI: 10.3389/fphar.2024.1308547
Development of a new hazard scoring system in primary neuronal cell cultures for drug-induced acute neuronal toxicity identification in early drug discovery
Abstract
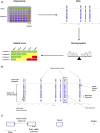
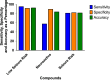
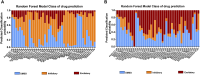
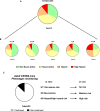
We investigated drug-induced acute neuronal electrophysiological changes using Micro-Electrode arrays (MEA) to rat primary neuronal cell cultures. Data based on 6-key MEA parameters were analyzed for plate-to-plate vehicle variability, effects of positive and negative controls, as well as data from over 100 reference drugs, mostly known to have pharmacological phenotypic and clinical outcomes. A Least Absolute Shrinkage and Selection Operator (LASSO) regression, coupled with expert evaluation helped to identify the 6-key parameters from many other MEA parameters to evaluate the drug-induced acute neuronal changes. Calculating the statistical tolerance intervals for negative-positive control effects on those 4-key parameters helped us to develop a new weighted hazard scoring system on drug-induced potential central nervous system (CNS) adverse effects (AEs). The weighted total score, integrating the effects of a drug candidate on the identified six-pivotal parameters, simply determines if the testing compound/concentration induces potential CNS AEs. Hereto, it uses four different categories of hazard scores: non-neuroactive, neuroactive, hazard, or high hazard categories. This new scoring system was successfully applied to differentiate the new compounds with or without CNS AEs, and the results were correlated with the outcome of in vivo studies in mice for one internal program. Furthermore, the Random Forest classification method was used to obtain the probability that the effect of a compound is either inhibitory or excitatory. In conclusion, this new neuronal scoring system on the cell assay is actively applied in the early de-risking of drug development and reduces the use of animals and associated costs.
Keywords: adverse effect (AE); hazard score system; micro-electrode array (MEA); neuronal cells; neuronal toxicity; seizures.
Copyright © 2024 Kreir, Putri, Tekle, Pibiri, d’Ydewalle, Van Ammel, Geys, Teisman, Gallacher and Lu.
Conflict of interest statement
All the authors were employed by company Janssen Research and Development, Janssen Pharmaceutical Companies of Johnson & Johnson.
Figures



References
LinkOut - more resources
Full Text Sources

