1H-NMR-based metabolomics reveals the preventive effect of Enteromorpha prolifera polysaccharides on diabetes in Zucker diabetic fatty rats
- PMID: 38873458
- PMCID: PMC11167149
- DOI: 10.1002/fsn3.4061
1H-NMR-based metabolomics reveals the preventive effect of Enteromorpha prolifera polysaccharides on diabetes in Zucker diabetic fatty rats
Abstract
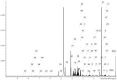
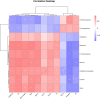
The primary objective of this investigation was to explore the beneficial impacts of Enteromorpha prolifera polysaccharide (EP) on dysglycemia in Zucker diabetic fatty (ZDF) rats, while also shedding light on its potential mechanism using 1H-NMR-based metabolomics. The results demonstrated a noteworthy reduction in fasting blood glucose (FBG, 46.3%), fasting insulin (50.17%), glycosylated hemoglobin A1c (HbA1c, 44.1%), and homeostatic model assessment of insulin resistance (HOMA-IR, 59.75%) following EP administration, while the insulin sensitivity index (ISI, 19.6%) and homeostatic model assessment of β-cell function (HOMA-β, 2.5-fold) were significantly increased. These findings indicate that EP enhances β-cell function, increases insulin sensitivity, and improves insulin resistance caused by diabetes. Moreover, EP significantly reduced serum lipid levels, suggesting improvement of dyslipidemia. Through the analysis of serum metabolomics, 17 metabolites were found to be altered in diabetic rats, 14 of which were upregulated and 3 of which were downregulated. Notably, the administration of EP successfully reversed the abnormal levels of 9 out of the 17 metabolites. Pathway analysis further revealed that EP treatment partially restored metabolic dysfunction, with notable effects observed in valine, leucine, and isoleucine metabolism; aminoacyl-transfer RNA (tRNA) biosynthesis; and ketone body metabolism. These findings collectively indicate the potential therapeutic efficacy of EP in preventing glycemic abnormalities and improving insulin resistance. Thus, EP holds promise as a valuable treatment option for individuals with diabetes.
Keywords: Enteromorpha prolifera polysaccharides; branched‐chain amino acid; glycemic abnormalities; metabolomics.
© 2024 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to exert influence on the research presented in this manuscript.
Figures





References
-
- Adams, S. H. , Hoppel, C. L. , Lok, K. H. , Zhao, L. , Wong, S. W. , Minkler, P. E. , Hwang, D. H. , Newman, J. W. , & Garvey, W. T. (2009). Plasma acylcarnitine profiles suggest incomplete long‐chain fatty acid beta‐oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African‐American women. The Journal of Nutrition, 139, 1073–1081. - PMC - PubMed
-
- Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein‐dye binding. Analytical Biochemistry, 72, 248–254. - PubMed
-
- Chen, K. , Wei, X. , Zhang, J. , Pariyani, R. , Jokioja, J. , Kortesniemi, M. , Linderborg, K. M. , Heinonen, J. , Sainio, T. , Zhang, Y. , & Yang, B. (2020). Effects of anthocyanin extracts from bilberry (Vaccinium myrtillus L.) and purple potato (Solanum tuberosum L. var. ‘Synkea Sakari’) on the plasma metabolomic profile of Zucker diabetic fatty rats. Journal of Agricultural and Food Chemistry, 68, 9436–9450. - PMC - PubMed
-
- Chen, L. , Ding, H. , Zhu, Y. , Guo, Y. , Tang, Y. , Xie, K. , Zhang, G. , Dai, G. , Gao, Y. , & Zhang, T. (2023). Untargeted and targeted metabolomics identify metabolite biomarkers for Salmonella enteritidis in chicken meat. Food Chemistry, 409, 135294. - PubMed
LinkOut - more resources
Full Text Sources

