Dihydromyricetin regulates KEAP1-Nrf2 pathways to enhance the survival of ischemic flap
- PMID: 38873488
- PMCID: PMC11167164
- DOI: 10.1002/fsn3.4049
Dihydromyricetin regulates KEAP1-Nrf2 pathways to enhance the survival of ischemic flap
Abstract
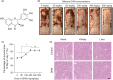
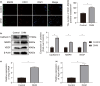
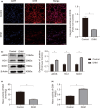
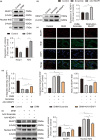
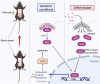
In clinical flap practice, there are a lot of studies being done on how to promote the survival of distal random flap necrosis in the hypoxic and ischemic state. As a traditional Chinese medicine, dihydromyricetin (DHM) is crucial in preventing oxidative stress and apoptosis in a number of disorders. In this work, we examined the impact of DHM on the ability to survive of ischemia flaps and looked into its fundamental mechanism. Our results showed that DHM significantly increased the ischemic flaps' survival area, encouraged angiogenesis and blood flow, reduced oxidative stress and apoptosis, and stimulated KEAP1-Nrf2 (Kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor) signaling pathways. Adeno-associated virus (AAV) upregulation of KEAP1 expression also negated the favorable effects of DHM on flap survival. By activating KEAP1-Nrf2 signaling pathways, DHM therapy promotes angiogenesis while reducing oxidative stress and apoptosis.
Keywords: angiogenesis; apoptosis; dihydromyricetin; ischemic flap; oxidative stress.
© 2024 The Authors. Food Science & Nutrition published by Wiley Periodicals LLC.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




Similar articles
-
Overexpression of miR-200a protects cardiomyocytes against hypoxia-induced apoptosis by modulating the kelch-like ECH-associated protein 1-nuclear factor erythroid 2-related factor 2 signaling axis.Int J Mol Med. 2016 Oct;38(4):1303-11. doi: 10.3892/ijmm.2016.2719. Epub 2016 Aug 26. Int J Mol Med. 2016. PMID: 27573160
-
Formononetin Improves the Survival of Random Skin Flaps Through PI3K/Akt-Mediated Nrf2 Antioxidant Defense System.Front Pharmacol. 2022 May 19;13:901498. doi: 10.3389/fphar.2022.901498. eCollection 2022. Front Pharmacol. 2022. PMID: 35662691 Free PMC article.
-
Dihydromyricetin inhibits oxidative stress and apoptosis in oxygen and glucose deprivation/reoxygenation‑induced HT22 cells by activating the Nrf2/HO‑1 pathway.Mol Med Rep. 2021 Jun;23(6):397. doi: 10.3892/mmr.2021.12036. Epub 2021 Mar 31. Mol Med Rep. 2021. PMID: 33786616
-
Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress.J Adv Res. 2021 Jul 6;34:43-63. doi: 10.1016/j.jare.2021.06.023. eCollection 2021 Dec. J Adv Res. 2021. PMID: 35024180 Free PMC article. Review.
-
Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases.J Tissue Eng Regen Med. 2020 Jun;14(6):869-883. doi: 10.1002/term.3053. Epub 2020 May 9. J Tissue Eng Regen Med. 2020. PMID: 32336035 Review.
Cited by
-
Organoid-Like Neurovascular Spheroids Promote the Recovery of Hypoxic-Ischemic Skin Flaps Through the Activation of Autophagy.Adv Healthc Mater. 2025 Jun;14(15):e2405154. doi: 10.1002/adhm.202405154. Epub 2025 Apr 16. Adv Healthc Mater. 2025. PMID: 40237031 Free PMC article.
-
Hypnea musciformis Seaweed Extract Protected Human Mesenchymal Stem Cells From Oxidative Stress Through NRF2 Activation.Food Sci Nutr. 2024 Nov 27;12(12):10816-10835. doi: 10.1002/fsn3.4615. eCollection 2024 Dec. Food Sci Nutr. 2024. PMID: 39723057 Free PMC article.
References
-
- Akhavani, M. A. , Sivakumar, B. , Paleolog, E. M. , & Kang, N. (2008). Angiogenesis and plastic surgery. Journal of Plastic, Reconstructive & Aesthetic Surgery, 61(12), 1425–1437. - PubMed
-
- Attinger, C. E. , & Colen, L. B. (2000). The role of microsurgical free flaps in foot and ankle surgery. Clinics in Podiatric Medicine and Surgery, 17(4), 649–680. - PubMed
-
- Bali, U. , Aydemir, I. , Keçeci, Y. , Yoleri, L. , & Tuğlu, M. İ. (2021). Effects of oxidative stress and apoptosis on vascularity and viability of perforator flaps. Biotechnic & Histochemistry, 96(7), 526–535. - PubMed
-
- Bardallo, R. G. , Panisello‐Roselló, A. , Sanchez‐Nuno, S. , Alva, N. , Roselló‐Catafau, J. , & Carbonell, T. (2022). Nrf2 and oxidative stress in liver ischemia/reperfusion injury. The FEBS Journal, 289(18), 5463–5479. - PubMed
-
- Brinkman, J. N. , Derks, L. H. , Klimek, M. , & Mureau, M. A. (2013). Perioperative fluid management and use of vasoactive and antithrombotic agents in free flap surgery: A literature review and clinical recommendations. Journal of Reconstructive Microsurgery, 29(6), 357–366. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

