Graphitic carbon nitride supported silver nanoparticles (AgNPs/g-C3N4): synthesis and photocatalytic behavior in the degradation of 2,4-dichlorophenoxyacetic acid
- PMID: 38873553
- PMCID: PMC11170562
- DOI: 10.1039/d4ra02658f
Graphitic carbon nitride supported silver nanoparticles (AgNPs/g-C3N4): synthesis and photocatalytic behavior in the degradation of 2,4-dichlorophenoxyacetic acid
Abstract
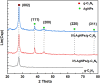
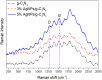
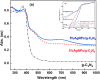
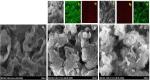
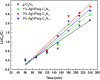
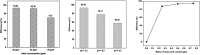
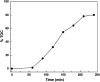
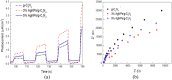
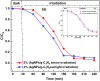
Graphitic carbon nitride supported silver nanoparticles (AgNPs/g-C3N4) with 1%, 3%, and 5% AgNPs were successfully synthesized by an "ex situ" method with ultrasound of a mixture of AgNP solution and g-C3N4. The AgNP solution was prepared by chemical reduction with trisodium citrate, and g-C3N4 was synthesized from the urea precursor. The supported nanoparticles were characterized by X-ray diffraction spectroscopy (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), nitrogen adsorption-desorption (BET), Fourier transformation infrared (FTIR) and Raman spectroscopy, ultraviolet-visible diffuse reflectance spectroscopy (UV-Vis DRS), photoluminescence spectroscopy (PL), electron paramagnetic resonance (EPR) and electrochemical impedance spectroscopy (EIS) Nyquist plots. The visible light-driven photocurrent measurement was performed by three on-off cycles of intermittent irradiation. The analyses show that AgNPs were evenly dispersed on g-C3N4, and have sizes ranging from 40 to 50 nm. The optical properties of the AgNPs/g-C3N4 material were significantly enhanced due to the plasmonic effect of AgNPs. The photocatalytic activity of catalysts was evaluated by 2,4-D degradation under visible light irradiation (λ > 420 nm). In the reaction conditions: pH 2.2; C o (2,4-D) 40 ppm; a m/v ratio of 0.5 g L-1, AgNPs/g-C3N4 materials exhibit superior photocatalytic activity compared to the pristine g-C3N4. The studies on the influence of free radicals and photogenerated holes, h+, show that ˙OH, O2˙-, and h+ play decisive roles in the photocatalytic activity of AgNPs/g-C3N4. The TOC result indicates the minimal toxicity of the by-products formed during the 2,4-D degradation. In addition, the AgNPs/g-C3N4 catalytic activity under direct sunlight irradiation was similar to that under artificial UV irradiation. Based on these results, a possible mechanism is proposed to explain the enhanced photocatalytic activity and stability of AgNPs/g-C3N4. Theoretical calculations on the interaction between 2,4-D and g-C3N4, Ag/g-C3N4 was also performed. The calculated results show that the adsorption of 2,4-D on Ag-modified g-C3N4 is significantly more effective compared to pristine g-C3N4.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures









References
-
- Walters J. Environmental fate of 2,3-dichlorophenoxyacetic acid. Environ. Monit. Pest. Manage. 2021;3510:1–18.
-
- Zuanazzi N. R. Ghisi N. d C. Oliveira E. C. Analysis of global trends and gaps for studies about 2,4-D herbicide toxicity: A scientometric review. Chemosphere. 2020;241:125016. - PubMed
-
- Hue H. K. Anh L. V. Trong D. B. Study of the adsorption of 2,4-dichlorophenoxyacetic acid from the aqueous solution onto activated carbon. Vietnam J. Chem. 2018;56(2):208–213.
-
- Goscianska J. Olejnik A. Removal of 2,4-D herbicide from aqueous solution by amino silane grafted mesoporous carbons. Adsorption. 2019;25:345–355.
-
- Shattar S. F. A. Zakaria N. A. Foo K. Y. Preparation of a montmorillonite-derived adsorbent for the practical treatment of ionic and nonionic pesticides. J. Mater. Res. Technol. 2019;8(5):4713–4724.
LinkOut - more resources
Full Text Sources

