Regulated vascular smooth muscle cell death in vascular diseases
- PMID: 38873710
- PMCID: PMC11533065
- DOI: 10.1111/cpr.13688
Regulated vascular smooth muscle cell death in vascular diseases
Abstract
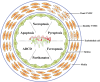
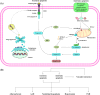
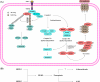
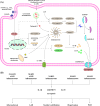
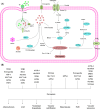
Regulated cell death (RCD) is a complex process that involves several cell types and plays a crucial role in vascular diseases. Vascular smooth muscle cells (VSMCs) are the predominant elements of the medial layer of blood vessels, and their regulated death contributes to the pathogenesis of vascular diseases. The types of regulated VSMC death include apoptosis, necroptosis, pyroptosis, ferroptosis, parthanatos, and autophagy-dependent cell death (ADCD). In this review, we summarize the current evidence of regulated VSMC death pathways in major vascular diseases, such as atherosclerosis, vascular calcification, aortic aneurysm and dissection, hypertension, pulmonary arterial hypertension, neointimal hyperplasia, and inherited vascular diseases. All forms of RCD constitute a single, coordinated cell death system in which one pathway can compensate for another during disease progression. Pharmacologically targeting RCD pathways has potential for slowing and reversing disease progression, but challenges remain. A better understanding of the role of regulated VSMC death in vascular diseases and the underlying mechanisms may lead to novel pharmacological developments and help clinicians address the residual cardiovascular risk in patients with cardiovascular diseases.
© 2024 The Author(s). Cell Proliferation published by Beijing Institute for Stem Cell and Regenerative Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Smooth muscle cell-driven vascular diseases and molecular mechanisms of VSMC plasticity.Cell Signal. 2018 Dec;52:48-64. doi: 10.1016/j.cellsig.2018.08.019. Epub 2018 Aug 30. Cell Signal. 2018. PMID: 30172025 Review.
-
Metabolism of vascular smooth muscle cells in vascular diseases.Am J Physiol Heart Circ Physiol. 2020 Sep 1;319(3):H613-H631. doi: 10.1152/ajpheart.00220.2020. Epub 2020 Aug 7. Am J Physiol Heart Circ Physiol. 2020. PMID: 32762559 Review.
-
Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis.Circulation. 2008 Oct 21;118(17):1748-57. doi: 10.1161/CIRCULATIONAHA.108.783738. Epub 2008 Oct 6. Circulation. 2008. PMID: 18838561
-
Cigarette smoke extract induces ferroptosis in vascular smooth muscle cells.Am J Physiol Heart Circ Physiol. 2020 Mar 1;318(3):H508-H518. doi: 10.1152/ajpheart.00559.2019. Epub 2020 Jan 24. Am J Physiol Heart Circ Physiol. 2020. PMID: 31975626
-
Gamut of glycolytic enzymes in vascular smooth muscle cell proliferation: Implications for vascular proliferative diseases.Biochim Biophys Acta Mol Basis Dis. 2024 Mar;1870(3):167021. doi: 10.1016/j.bbadis.2024.167021. Epub 2024 Jan 10. Biochim Biophys Acta Mol Basis Dis. 2024. PMID: 38216067
Cited by
-
Berberine Inhibits Abdominal Aortic Aneurysm Formation and Vascular Smooth Muscle Cell Phenotypic Switching by Regulating the Nrf2 Pathway.J Cell Mol Med. 2025 Apr;29(7):e70509. doi: 10.1111/jcmm.70509. J Cell Mol Med. 2025. PMID: 40193135 Free PMC article.
-
Aortic Aneurysm with and without Dissection and Concomitant Atherosclerosis-Differences in a Retrospective Study.J Cardiovasc Dev Dis. 2024 Oct 8;11(10):311. doi: 10.3390/jcdd11100311. J Cardiovasc Dev Dis. 2024. PMID: 39452282 Free PMC article.
-
Class III Phosphatidylinositol-3 Kinase/Vacuolar Protein Sorting 34 in Cardiovascular Health and Disease.J Cardiovasc Transl Res. 2025 Apr;18(2):392-407. doi: 10.1007/s12265-024-10581-z. Epub 2025 Jan 16. J Cardiovasc Transl Res. 2025. PMID: 39821606 Free PMC article. Review.
-
ALOX5 regulates vascular smooth muscle cells pyroptosis to affect abdominal aortic aneurysm formation.Sci Rep. 2025 Aug 9;15(1):29123. doi: 10.1038/s41598-025-14268-6. Sci Rep. 2025. PMID: 40781109 Free PMC article.
-
Multi-Omic Insight Into the Molecular Networks in the Pathogenesis of Coronary Artery Disease.J Am Heart Assoc. 2025 Apr;14(7):e037203. doi: 10.1161/JAHA.124.037203. Epub 2025 Mar 26. J Am Heart Assoc. 2025. PMID: 40135555 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- 82270454/National Natural Science Foundation of China
- 82000299/National Natural Science Foundation of China
- 82100292/National Natural Science Foundation of China
- 82070436/National Natural Science Foundation of China
- ZNQNJC2023001/Youth Interdisciplinary Special Fund of Zhongnan Hospital of Wuhan University
LinkOut - more resources
Full Text Sources
Medical

