Dapagliflozin for Critically Ill Patients With Acute Organ Dysfunction: The DEFENDER Randomized Clinical Trial
- PMID: 38873723
- PMCID: PMC11304119
- DOI: 10.1001/jama.2024.10510
Dapagliflozin for Critically Ill Patients With Acute Organ Dysfunction: The DEFENDER Randomized Clinical Trial
Abstract
Importance: Sodium-glucose cotransporter 2 (SGLT-2) inhibitors improve outcomes in patients with type 2 diabetes, heart failure, and chronic kidney disease, but their effect on outcomes of critically ill patients with organ failure is unknown.
Objective: To determine whether the addition of dapagliflozin, an SGLT-2 inhibitor, to standard intensive care unit (ICU) care improves outcomes in a critically ill population with acute organ dysfunction.
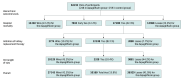
Design, setting, and participants: Multicenter, randomized, open-label, clinical trial conducted at 22 ICUs in Brazil. Participants with unplanned ICU admission and presenting with at least 1 organ dysfunction (respiratory, cardiovascular, or kidney) were enrolled between November 22, 2022, and August 30, 2023, with follow-up through September 27, 2023.
Intervention: Participants were randomized to 10 mg of dapagliflozin (intervention, n = 248) plus standard care or to standard care alone (control, n = 259) for up to 14 days or until ICU discharge, whichever occurred first.
Main outcomes and measures: The primary outcome was a hierarchical composite of hospital mortality, initiation of kidney replacement therapy, and ICU length of stay through 28 days, analyzed using the win ratio method. Secondary outcomes included the individual components of the hierarchical outcome, duration of organ support-free days, ICU, and hospital stay, assessed using bayesian regression models.
Results: Among 507 randomized participants (mean age, 63.9 [SD, 15] years; 46.9%, women), 39.6% had an ICU admission due to suspected infection. The median time from ICU admission to randomization was 1 day (IQR, 0-1). The win ratio for dapagliflozin for the primary outcome was 1.01 (95% CI, 0.90 to 1.13; P = .89). Among all secondary outcomes, the highest probability of benefit found was 0.90 for dapagliflozin regarding use of kidney replacement therapy among 27 patients (10.9%) in the dapagliflozin group vs 39 (15.1%) in the control group.
Conclusion and relevance: The addition of dapagliflozin to standard care for critically ill patients and acute organ dysfunction did not improve clinical outcomes; however, confidence intervals were wide and could not exclude relevant benefits or harms for dapagliflozin.
Trial registration: ClinicalTrials.gov Identifier: NCT05558098.
Conflict of interest statement
Figures


Comment in
-
Sodium-Glucose Cotransporter 2 Therapy for Acute Organ Dysfunction in Critically Ill Patients.JAMA. 2024 Aug 6;332(5):377-379. doi: 10.1001/jama.2024.10171. JAMA. 2024. PMID: 38873705 No abstract available.
References
-
- Zelniker TA, Wiviott SD, Raz I, et al. SGLT2 inhibitors for primary and secondary prevention of cardiovascular and renal outcomes in type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet. 2019;393(10166):31-39. doi: 10.1016/S0140-6736(18)32590-X - DOI - PubMed
-
- Nuffield Department of Population Health Renal Studies Group; SGLT2 inhibitor Meta-Analysis Cardio-Renal Trialists’ Consortium . Impact of diabetes on the effects of sodium glucose co-transporter-2 inhibitors on kidney outcomes: collaborative meta-analysis of large placebo-controlled trials. Lancet. 2022;400(10365):1788-1801. doi: 10.1016/S0140-6736(22)02074-8 - DOI - PMC - PubMed
-
- Kosiborod MN, Esterline R, Furtado RHM, et al. Dapagliflozin in patients with cardiometabolic risk factors hospitalised with COVID-19 (DARE-19): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021;9(9):586-594. doi: 10.1016/S2213-8587(21)00180-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

