TAF15 downregulation contributes to the benefits of physical training on dendritic spines and working memory in aged mice
- PMID: 38874013
- PMCID: PMC11488317
- DOI: 10.1111/acel.14244
TAF15 downregulation contributes to the benefits of physical training on dendritic spines and working memory in aged mice
Abstract
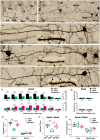
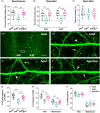
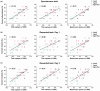
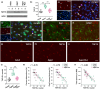
Moderate physical training has been shown to hinder age-related memory decline. While the benefits of physical training on hippocampal memory function are well-documented, little is known about its impact on working memory, which is linked to the prelimbic cortex (PrL), one major subdivision of the prefrontal cortex. Here, we examined the effects of physical training on spatial working memory in a well-established animal model of physical training, starting at 16 months of age and continuing for 5 months (running wheel 1 h/day and 5 days/week). This training strategy improved spatial working memory in aged mice (22-month-old), which was accompanied by an increased spine density and a lower TAF15 expression in the PrL. Specifically, physical training affected both thin and mushroom-type spines on PrL pyramidal cells, and prevented age-related loss of spines on selective segments of apical dendritic branches. Correlation analysis revealed that increased TAF15-expression was detrimental to the dendritic spines. However, physical training downregulated TAF15 expression in the PrL, preserving the dendritic spines on PrL pyramidal cells and improving working memory in trained aged mice. When TAF15 was overexpressed in the PrL via a viral approach, the benefits of physical training on the dendritic spines and working memory were abolished. These data suggest that physical training at a moderate pace might downregulate TAF15 expression in the PrL, which favors the dendritic spines on PrL pyramidal cells, thereby improving spatial working memory.
Keywords: TAF15; aging; physical running; prefrontal cortex; spine; working memory.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures

Similar articles
-
Loss of spines in the prelimbic cortex is detrimental to working memory in mice with early-life adversity.Mol Psychiatry. 2023 Aug;28(8):3444-3458. doi: 10.1038/s41380-023-02197-7. Epub 2023 Jul 27. Mol Psychiatry. 2023. PMID: 37500828 Free PMC article.
-
Selective Loss of Thin Spines in Area 7a of the Primate Intraparietal Sulcus Predicts Age-Related Working Memory Impairment.J Neurosci. 2018 Dec 5;38(49):10467-10478. doi: 10.1523/JNEUROSCI.1234-18.2018. Epub 2018 Oct 24. J Neurosci. 2018. PMID: 30355632 Free PMC article.
-
Running-induced memory enhancement correlates with the preservation of thin spines in the hippocampal area CA1 of old C57BL/6 mice.Neurobiol Aging. 2017 Apr;52:106-116. doi: 10.1016/j.neurobiolaging.2017.01.002. Epub 2017 Jan 11. Neurobiol Aging. 2017. PMID: 28157554
-
Plastic changes to dendritic spines in the cerebellar and prefrontal cortices underlie the decline in motor coordination and working memory during successful aging.Behav Brain Res. 2021 Feb 26;400:113014. doi: 10.1016/j.bbr.2020.113014. Epub 2020 Dec 10. Behav Brain Res. 2021. PMID: 33309738
-
Interactions between estradiol, BDNF and dendritic spines in promoting memory.Neuroscience. 2013 Jun 3;239:34-45. doi: 10.1016/j.neuroscience.2012.10.019. Epub 2012 Oct 16. Neuroscience. 2013. PMID: 23079626 Free PMC article. Review.
References
-
- Aoki, N. , Higashi, S. , Kawakami, I. , Kobayashi, Z. , Hosokawa, M. , Katsuse, O. , Togo, T. , Hirayasu, Y. , & Akiyama, H. (2012). Localization of fused in sarcoma (FUS) protein to the post‐synaptic density in the brain. Acta Neuropathologica, 124(3), 383–394. 10.1007/s00401-012-0984-6 - DOI - PubMed
-
- Bloss, E. B. , Janssen, W. G. , Ohm, D. T. , Yuk, F. J. , Wadsworth, S. , Saardi, K. M. , McEwen, B. S. , & Morrison, J. H. (2011). Evidence for reduced experience‐dependent dendritic spine plasticity in the aging prefrontal cortex. The Journal of Neuroscience, 31(21), 7831–7839. 10.1523/JNEUROSCI.0839-11.2011 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

