2‑D08 mediates notable anticancer effects through multiple cellular pathways in uterine leiomyosarcoma cells
- PMID: 38874019
- PMCID: PMC11200159
- DOI: 10.3892/or.2024.8756
2‑D08 mediates notable anticancer effects through multiple cellular pathways in uterine leiomyosarcoma cells
Abstract
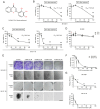
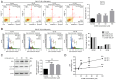
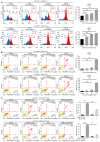
2',3',4'‑trihydroxyflavone (2‑D08), a SUMO E2 inhibitor, has several biological functions, including anticancer activity, but its effects on uterine leiomyosarcoma (Ut‑LMS) are unknown. The anticancer activity of 2‑D08 was explored in an in vitro model using SK‑LMS‑1 and SK‑UT‑1B cells (human Ut‑LMS cells). Treatment with 2‑D08 inhibited cell viability in a dose‑ and time‑dependent manner and significantly inhibited the colony‑forming ability of Ut‑LMS cells. In SK‑UT‑1B cells treated with 2‑D08, flow cytometric analysis revealed a slight increase in apoptotic rates, while cell cycle progression remained unaffected. Western blotting revealed elevated levels of RIP1, indicating induction of necrosis, but LC3B levels remained unchanged, suggesting no effect on autophagy. A lactate dehydrogenase (LDH) assay confirmed increased LDH release, further supporting the induction of apoptosis and necrosis by 2‑D08 in SK‑UT‑1B cells. 2‑D08‑induced production of reactive oxygen species and apoptosis progression were observed in SK‑LMS‑1 cells. Using Ki67 staining and bromodeoxyuridine assays, it was found that 2‑D08 suppressed proliferation in SK‑LMS‑1 cells, while treatment for 48 h led to cell‑cycle arrest. 2‑D08 upregulated p21 protein expression in SK‑LMS‑1 cells and promoted apoptosis through caspase‑3. Evaluation of α‑SM‑actin, calponin 1 and TAGLN expression indicated that 2‑D08 did not directly initiate smooth muscle phenotypic switching in SK‑LMS‑1 cells. Transcriptome analysis on 2‑D08‑treated SK‑LMS‑1 cells identified significant differences in gene expression and suggested that 2‑D08 modulates cell‑cycle‑ and apoptosis‑related pathways. The analysis identified several differentially expressed genes and significant enrichment for biological processes related to DNA replication and molecular functions associated with the apoptotic process. It was concluded that 2‑D08 exerts antitumor effects in Ut‑LMS cells by modulating multiple signaling pathways and that 2‑D08 may be a promising candidate for the treatment of human Ut‑LMS. The present study expanded and developed knowledge regarding Ut‑LMS management and indicated that 2‑D08 represents a notable finding in the exploration of fresh treatment options for such cancerous tumors.
Keywords: 2‑D08; SK‑LMS‑1; SK‑UT‑1B; apoptosis; leiomyosarcoma; proliferation; uterine.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
-
- Chern JY, Boyd LR, Blank SV. Uterine sarcomas: The latest approaches for these rare but potentially deadly tumors. Oncology (Williston Park) 2017;31:229–236. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

