Elucidating the pivotal molecular mechanisms, therapeutic and neuroprotective effects of lithium in traumatic brain injury
- PMID: 38874089
- PMCID: PMC11177180
- DOI: 10.1002/brb3.3595
Elucidating the pivotal molecular mechanisms, therapeutic and neuroprotective effects of lithium in traumatic brain injury
Abstract
Introduction: Traumatic brain injury (TBI) refers to damage to brain tissue by mechanical or blunt force via trauma. TBI is often associated with impaired cognitive abilities, like difficulties in memory, learning, attention, and other higher brain functions, that typically remain for years after the injury. Lithium is an elementary light metal that is only utilized in salt form due to its high intrinsic reactivity. This current review discusses the molecular mechanisms and therapeutic and neuroprotective effects of lithium in TBI.
Method: The "Boolean logic" was used to search for articles on the subject matter in PubMed and PubMed Central, as well as Google Scholar.
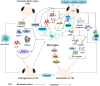
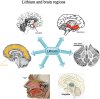
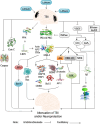
Results: Lithium's therapeutic action is extremely complex, involving multiple effects on gene secretion, neurotransmitter or receptor-mediated signaling, signal transduction processes, circadian modulation, as well as ion transport. Lithium is able to normalize multiple short- as well as long-term modifications in neuronal circuits that ultimately result in disparity in cortical excitation and inhibition activated by TBI. Also, lithium levels are more distinct in the hippocampus, thalamus, neo-cortex, olfactory bulb, amygdala as well as the gray matter of the cerebellum following treatment of TBI.
Conclusion: Lithium attenuates neuroinflammation and neuronal toxicity as well as protects the brain from edema, hippocampal neurodegeneration, loss of hemispheric tissues, and enhanced memory as well as spatial learning after TBI.
Keywords: TBI; bipolar disorder; lithium; neuroinflammation; neuroprotection; neurotransmission.
© 2024 The Author(s). Brain and Behavior published by Wiley Periodicals LLC.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
References
-
- Ahmad, M. , Rose, M. E. , Vagni, V. , Griffith, R. P. , Dixon, C. E. , Kochanek, P. M. , Hickey, R. W. , & Graham, S. H. (2008). Genetic disruption of cyclooxygenase‐2 does not improve histological or behavioral outcome after traumatic brain injury in mice. Journal of Neuroscience Research, 86(16), 3605–3612. 10.1002/jnr.21809 - DOI - PMC - PubMed
-
- Bachmann, R. F. , Wang, Y. , Yuan, P. , Zhou, R. , Li, X. , Alesci, S. , Du, J. , & Manji, H. K. (2009). Common effects of lithium and valproate on mitochondrial functions: Protection against methamphetamine‐induced mitochondrial damage. The International Journal of Neuropsychopharmacology, 12(6), 805–822. 10.1017/S1461145708009802 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

