Smart Microneedle Arrays Integrating Cell-Free Therapy and Nanocatalysis to Treat Liver Fibrosis
- PMID: 38874114
- PMCID: PMC11336984
- DOI: 10.1002/advs.202309940
Smart Microneedle Arrays Integrating Cell-Free Therapy and Nanocatalysis to Treat Liver Fibrosis
Abstract
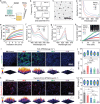
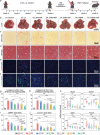
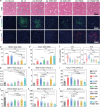
Liver fibrosis is a chronic pathological condition lacking specific clinical treatments. Stem cells, with notable potential in regenerative medicine, offer promise in treating liver fibrosis. However, stem cell therapy is hindered by potential immunological rejection, carcinogenesis risk, efficacy variation, and high cost. Stem cell secretome-based cell-free therapy offers potential solutions to address these challenges, but it is limited by low delivery efficiency and rapid clearance. Herein, an innovative approach for in situ implantation of smart microneedle (MN) arrays enabling precisely controlled delivery of multiple therapeutic agents directly into fibrotic liver tissues is developed. By integrating cell-free and platinum-based nanocatalytic combination therapy, the MN arrays can deactivate hepatic stellate cells. Moreover, they promote excessive extracellular matrix degradation by more than 75%, approaching normal levels. Additionally, the smart MN arrays can provide hepatocyte protection while reducing inflammation levels by ≈70-90%. They can also exhibit remarkable capability in scavenging almost 100% of reactive oxygen species and alleviating hypoxia. Ultimately, this treatment strategy can effectively restrain fibrosis progression. The comprehensive in vitro and in vivo experiments, supplemented by proteome and transcriptome analyses, substantiate the effectiveness of the approach in treating liver fibrosis, holding immense promise for clinical applications.
Keywords: controlled release; liver fibrosis; microneedle array; nanozyme; stem cell secretome.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Collagenase Type I and Probucol-Loaded Nanoparticles Penetrate the Extracellular Matrix to Target Hepatic Stellate Cells for Hepatic Fibrosis Therapy.Acta Biomater. 2024 Feb;175:262-278. doi: 10.1016/j.actbio.2023.12.027. Epub 2023 Dec 21. Acta Biomater. 2024. PMID: 38141933
-
Regenerative Potential of Mesenchymal Stem Cells' (MSCs) Secretome for Liver Fibrosis Therapies.Int J Mol Sci. 2021 Dec 10;22(24):13292. doi: 10.3390/ijms222413292. Int J Mol Sci. 2021. PMID: 34948088 Free PMC article. Review.
-
Collagenase-I decorated co-delivery micelles potentiate extracellular matrix degradation and hepatic stellate cell targeting for liver fibrosis therapy.Acta Biomater. 2022 Oct 15;152:235-254. doi: 10.1016/j.actbio.2022.08.065. Epub 2022 Sep 8. Acta Biomater. 2022. PMID: 36087869
-
The anti-fibrotic effect of human fetal skin-derived stem cell secretome on the liver fibrosis.Stem Cell Res Ther. 2020 Sep 3;11(1):379. doi: 10.1186/s13287-020-01891-5. Stem Cell Res Ther. 2020. PMID: 32883340 Free PMC article.
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
Cited by
-
Engineering therapeutical extracellular vesicles for clinical translation.Trends Biotechnol. 2025 Jan;43(1):61-82. doi: 10.1016/j.tibtech.2024.08.007. Epub 2024 Sep 2. Trends Biotechnol. 2025. PMID: 39227240 Review.
-
Amphiphilic lipid-peptide engineered placenta-derived mesenchymal stem cells for liver fibrosis treatment.Asian J Pharm Sci. 2025 Aug;20(4):101061. doi: 10.1016/j.ajps.2025.101061. Epub 2025 Apr 22. Asian J Pharm Sci. 2025. PMID: 40703618 Free PMC article.
References
-
- a) Xu M., Xu H.‐H., Lin Y., Sun X., Wang L.‐J., Fang Z.‐P., Su X.‐H., Liang X.‐J., Hu Y., Liu Z.‐M., Cheng Y., Wei Y., Li J., Li L., Liu H.‐J., Cheng Z., Tang N., Peng C., Li T., Liu T., Qiao L., Wu D., Ding Y.‐Q., Zhou W.‐J., Cell 2019, 178, 1478; - PubMed
- b) Kisseleva T., Brenner D., Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151; - PubMed
- c) Zhao M., Wang L., Wang M., Zhou S., Lu Y., Cui H., Racanelli A. C., Zhang L., Ye T., Ding B., Zhang B., Yang J., Yao Y., Signal Transduct. Target. Ther. 2022, 7, 206. - PMC - PubMed
-
- a) Kim E. R., Park J. S., Kim J. H., Oh J. Y., Oh I. J., Choi D. H., Lee Y., Park I. S., Kim S., Lee D. H., Cheon J. H., Bae J. W., Lee M., Cho J. W., An I. B., Nam E. J., Yang S. I., Lee M. S., Bae S. H., Lee Y. h., Hepatology 2022, 75, 1523; - PubMed
- b) Sharma S., Guillou D. L., Chen J. Y., Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 662. - PubMed
MeSH terms
Grants and funding
- 2019YFA0111300/National Key Research and Development Program of China
- 52373166/National Natural Science Foundation of China
- 22277155/National Natural Science Foundation of China
- 2024B1515020025/Natural Science Foundation of Guangdong Province for Distinguished Young Scholar
- 2024A04J6572/Science and Technology Program of Guangzhou
LinkOut - more resources
Full Text Sources
Medical
