Extracellular Vesicle Preparation and Analysis: A State-of-the-Art Review
- PMID: 38874129
- PMCID: PMC11321646
- DOI: 10.1002/advs.202401069
Extracellular Vesicle Preparation and Analysis: A State-of-the-Art Review
Abstract
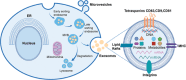
In recent decades, research on Extracellular Vesicles (EVs) has gained prominence in the life sciences due to their critical roles in both health and disease states, offering promising applications in disease diagnosis, drug delivery, and therapy. However, their inherent heterogeneity and complex origins pose significant challenges to their preparation, analysis, and subsequent clinical application. This review is structured to provide an overview of the biogenesis, composition, and various sources of EVs, thereby laying the groundwork for a detailed discussion of contemporary techniques for their preparation and analysis. Particular focus is given to state-of-the-art technologies that employ both microfluidic and non-microfluidic platforms for EV processing. Furthermore, this discourse extends into innovative approaches that incorporate artificial intelligence and cutting-edge electrochemical sensors, with a particular emphasis on single EV analysis. This review proposes current challenges and outlines prospective avenues for future research. The objective is to motivate researchers to innovate and expand methods for the preparation and analysis of EVs, fully unlocking their biomedical potential.
Keywords: artificial intelligence; disease diagnosis; extracellular vesicles; single EV.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Trams E. G., Lauter C. J., Salem N. Jr., Heine U., Biochimica et Biophysica Acta (BBA) – Biomembranes 1981, 645, 63. - PubMed
-
- Johnstone R. M., Adam M., Hammond J. R., Orr L., Turbide C., J. Biol. Chem. 1987, 262, 9412. - PubMed
-
- Thery C., Witwer K. W., Aikawa E., Alcaraz M. J., Anderson J. D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin‐Smith G. K., Ayre D. C., Bach J. M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N. N., Baxter A. A., Bebawy M., Beckham C., Bedina Zavec A., Benmoussa A., Berardi A. C., Bergese P., Bielska E., J Extracell Vesicles 2018, 7, 1535750. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
