Tumor-like conditions that mimic liver tumors
- PMID: 38874132
- PMCID: PMC12239536
- DOI: 10.4274/dir.2024.242826
Tumor-like conditions that mimic liver tumors
Abstract
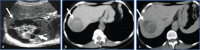
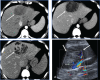
Non-neoplastic tumor-like conditions of the liver can appear similar to hepatic neoplasms. In many cases, a biopsy is required to confirm the pathology. However, several tumor-like conditions can be correctly diagnosed or suggested prospectively, thus saving patients from unnecessary anxiety and expense. In this image-focused review, we present the ultrasound, computed tomography, magnetic resonance imaging, and positron emission tomography scan features of eight such entities. Clues that indicate the correct pathology are discussed, and the usual clinical setting is described. Many of these lesions are treated differently from true neoplasms, and the current treatment plan is discussed in many of the cases presented. After reviewing this article, the reader will have a better understanding of these lesions and the situations in which they should be included in the differential diagnosis.
Keywords: Benign hepatic lesion; hepatic amyloidosis; hepatic extramedullary hematopoiesis; hepatic pseudotumor; hepatic sarcoidosis; hepatic tumor mimics; hepatic tumor-like conditions; hepatobiliary tuberculomas; liver imaging; mesenchymal hamartoma; myofibroblastoma; peliosis hepatis.
Conflict of interest statement
The authors declared no conflicts of interest.
Figures











References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

