diABZI and poly(I:C) inhibit osteoclastic bone resorption by inducing IRF7 and IFIT3
- PMID: 38874138
- PMCID: PMC11337579
- DOI: 10.1093/jbmr/zjae093
diABZI and poly(I:C) inhibit osteoclastic bone resorption by inducing IRF7 and IFIT3
Abstract
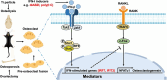
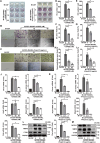
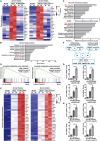
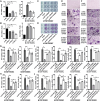
Type I interferons (IFN-I) are pleiotropic factors endowed with multiple activities that play important roles in innate and adaptive immunity. Although many studies indicate that IFN-I inducers exert favorable effects on broad-spectrum antivirus, immunomodulation, and anti-tumor activities by inducing endogenous IFN-I and IFN-stimulated genes, their function in bone homeostasis still needs further exploration. Here, our study demonstrates 2 distinct IFN-I inducers, diABZI and poly(I:C), as potential therapeutics to alleviate osteolysis and osteoporosis. First, IFN-I inducers suppress the genes that control osteoclast (OC) differentiation and activity in vitro. Moreover, diABZI alleviates bone loss in Ti particle-induced osteolysis and ovariectomized -induced osteoporosis in vivo by inhibiting OC differentiation and function. In addition, the inhibitory effects of IFN-I inducers on OC differentiation are not observed in macrophages derived from Ifnar1-/-mice, which indicate that the suppressive effect of IFN-I inducers on OC is IFNAR-dependent. Mechanistically, RNAi-mediated silencing of IRF7 and IFIT3 in OC precursors impairs the suppressive effect of the IFN-I inducers on OC differentiation. Taken together, these results demonstrate that IFN-I inducers play a protective role in bone turnover by limiting osteoclastogenesis and bone resorption through the induction of OC-specific mediators via the IFN-I signaling pathway.
Keywords: IFIT3; IFN-I inducer; IRF7; ISG mediator; bone resorption; osteoclast; osteolysis; osteoporosis.
Plain language summary
OCs are responsible for bone resorption, and their excessive differentiation and enhanced activity will lead to bone resorption diseases such as osteoporosis and osteolysis. Here, our study demonstrates 2 distinct IFN-I inducers, diABZI and poly(I:C), as potential therapeutics to alleviate osteolysis and osteoporosis. IFN-I inducers suppress OC differentiation, and particularly diABZI alleviates bone loss in osteolysis and osteoporosis mouse models. Taken together, IFN-I inducers play a protective role in bone turnover by limiting osteoclastogenesis and bone resorption through the induction of OC-specific mediators via the IFN-I signaling pathway. Our in-depth and comprehensive discovery of the IFN-I inducer would provide new insight into OC biology and therapeutic targets for osteoclastic bone resorption diseases.
© The Author(s) 2024. Published by Oxford University Press on behalf of the American Society for Bone and Mineral Research.
Conflict of interest statement
All authors state that they have no conflicts of interest.
Figures



Similar articles
-
Dendritic cells-derived interferon-λ1 ameliorated inflammatory bone destruction through inhibiting osteoclastogenesis.Cell Death Dis. 2020 Jun 2;11(6):414. doi: 10.1038/s41419-020-2612-z. Cell Death Dis. 2020. PMID: 32488049 Free PMC article.
-
Alexidine Dihydrochloride Attenuates Osteoclast Formation and Bone Resorption and Protects Against LPS-Induced Osteolysis.J Bone Miner Res. 2016 Mar;31(3):560-72. doi: 10.1002/jbmr.2710. Epub 2016 Jan 6. J Bone Miner Res. 2016. PMID: 26363136
-
Dual effects of baicalin on osteoclast differentiation and bone resorption.J Cell Mol Med. 2018 Oct;22(10):5029-5039. doi: 10.1111/jcmm.13785. Epub 2018 Jul 16. J Cell Mol Med. 2018. PMID: 30010244 Free PMC article.
-
The Dramatic Role of IFN Family in Aberrant Inflammatory Osteolysis.Curr Gene Ther. 2021;21(2):112-129. doi: 10.2174/1566523220666201127114845. Curr Gene Ther. 2021. PMID: 33245272 Review.
-
Beyond resorption: osteoclasts as drivers of bone formation.Cell Regen. 2024 Oct 11;13(1):22. doi: 10.1186/s13619-024-00205-x. Cell Regen. 2024. PMID: 39392536 Free PMC article. Review.
Cited by
-
Ubiquitination of NS1 Confers Differential Adaptation of Zika Virus in Mammalian Hosts and Mosquito Vectors.Adv Sci (Weinh). 2024 Oct;11(39):e2408024. doi: 10.1002/advs.202408024. Epub 2024 Aug 19. Adv Sci (Weinh). 2024. PMID: 39159062 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2018YFA0900803/National Key Research and Development Program of China
- 82301982/National Natural Science Foundation of China
- BK20230281/Natural Science Foundation of Jiangsu Province
- 2021-I2M-1-041/CAMS Initiation Fund for Medical Sciences
- 2019PT310028/Non-profit Central Research Institute Fund of CAMS
LinkOut - more resources
Full Text Sources

