NRG-BN002: Phase I study of ipilimumab, nivolumab, and the combination in patients with newly diagnosed glioblastoma
- PMID: 38874333
- PMCID: PMC11376446
- DOI: 10.1093/neuonc/noae058
NRG-BN002: Phase I study of ipilimumab, nivolumab, and the combination in patients with newly diagnosed glioblastoma
Abstract
Background: Immune checkpoint inhibitors (ICIs) have efficacy in several solid tumors but limited efficacy in glioblastoma (GBM). This study evaluated the safety of anti-CTLA-4 and anti-PD-1 ICIs alone or in combination in newly diagnosed GBM after completion of standard radiochemotherapy with the subsequent intent to test combinatorial ICIs in this setting.
Methods: The primary endpoint was dose-limiting toxicity (DLT) for adults with unifocal, supratentorial newly diagnosed GBM after resection and chemoradiation. Ipilimumab and nivolumab were tested separately and in combination with a planned expansion cohort dependent upon DLT results.
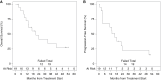
Results: Thirty-two patients were enrolled at 9 institutions: 6 to each DLT assessment cohort and 14 to the expansion cohort. Median age: 55 years, 67.7% male, 83.9% White. Treatment was well tolerated with 16% Grade 4 events; the combination did not have unexpectedly increased toxicity, with no Grade 5 events. One DLT was seen in each single-agent treatment; none were observed in the combination, leading to expanded accrual of the combined treatment. The median follow-up was 19.6 months. For all patients receiving combination treatment, median overall survival (OS) and progression-free survival (PFS) were 20.7 and 16.1 months, respectively.
Conclusions: IPI and NIVO are safe and tolerable with toxicities similar to those noted with other cancers when given in combination with adjuvant temozolomide for newly diagnosed GBM. Combination IPI + NIVO is not substantially more toxic than single agents. These results support a subsequent efficacy trial to test the combination of ICIs in Phase II/III for patients with newly diagnosed GBM.
Clinicaltrials.gov registration: NCT02311920.
Keywords: GBM; Phase I; immune checkpoint inhibitor; ipilimumab; nivolumab.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
M.W.G., K.A., N.B., R.R.R., A.D.V., and C.K.-M. report no conflicts of interest. K.W. and M.W reports payment to institution, NRG Oncology SDMC grant from NCI. S.C. reports salary support from institutional K12 grant: NIH K12CA076917, and participation on a local advisory board for Seagen Inc on Her2 + brain metastases on October 14, 2021. F.M.I. reports grants paid to the doctor’s institution from Merck, Bristol Myers Squibb, Roche, Sapience, Novocure, Celldex, Tocagen, Forma, Celldex, and Northwest Biotherapeutics, Sapience. F.M.I. reports consulting fees received from Novocure, Regeneron, Tocagen, Alexion Pharmaceuticals, Abbvie, Guidepoint Global, Merck, Kiyatec, PPD, Massive Bio, Medtronic, MimiVax, Gennao Bio, and Xcures; support for attending meetings/travel from Roche/Genentech and Oncoceutics; 2 US provisional patent applications (62/739,617 and 63/062,805) through Columbia University; participation on Advarra (Mimivax) Data Safety Monitoring Board; and stock in Praesidia Bio. A.E.S. reports a Merck Pharmaceuticals grant for another unrelated study; no personal funding was received for this trial. A.E.S. reports executive committee role for the Joint Section on Tumors of the AANS & CNS. P.Y.W. reports research support from AstraZeneca/Medimmune, BeiGene, Celgene, Chimerix, Eli Lily, Genentech/Roche, Kazia, MediciNova, Merck, Novartis, Nuvation Bio, Puma, Servier, Vascular Biogenics, and VBI Vaccines; and serves on advisory boards for AstraZeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, GlaxoSmithKline, Insightec, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, and VBI Vaccines; participation on Data Safety Monitoring Board or Advisory Board for Astra Zeneca, Bayer, Black Diamond, Boehringer Ingelheim, Boston Pharmaceuticals, Celularity, Chimerix, Day One Bio, Genenta, Glaxo Smith Kline, Karyopharm, Merck, Mundipharma, Novartis, Novocure, Nuvation Bio, Prelude Therapeutics, Sapience, Servier, Sagimet, Vascular Biogenics, VBI Vaccines. M.P.M. reports consulting fees from Kazia, Karyopharm, Sapience, ZapX, Xoft, and Tocagen; Participation on a Data Safety Monitoring Board or Advisory Board for Mevion; leadership or fiduciary role with Xcision (unpaid) and Oncoceutics, and Chimerix stock.
Figures
References
-
- Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups Radiotherapy Groups, National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical