Color-Tunable Room-Temperature Phosphorescence from Non-Aromatic-Polymer-Involved Charge Transfer
- PMID: 38874342
- PMCID: PMC11321690
- DOI: 10.1002/advs.202404698
Color-Tunable Room-Temperature Phosphorescence from Non-Aromatic-Polymer-Involved Charge Transfer
Abstract
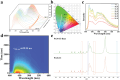
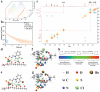
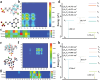
Polymeric room-temperature phosphorescence (RTP) materials especially multicolor RTP systems hold great promise in concrete applications. A key feature in these applications is a triplet charge transfer transition. Aromatic electron donors and electron acceptors are often essential to ensure persistent RTP. There is much interest in fabricating non-aromatic charge-transfer-mediated RTP materials and it still remains a formidable challenge to achieve color-tunable RTP via charge transfer. Herein, a charge-transfer-mediated RTP material by embedding quinoline derivatives within a non-aromatic polymer matrix such as polyacrylamide (PAM) or polyvinyl alcohol (PVA) is developed. Through-space charge transfer (TSCT) is achieved upon alkali- or heat treatment to realize a long phosphorescence lifetime of up to 629.90 ms, high phosphorescence quantum yield of up to 20.51%, and a green-to-blue afterglow for more than 20 s at room temperature. This color-tunable RTP emerges from a nonaromatic polymer to single phosphor charge transfer that has rarely been reported before. This finding suggests that an effective and simple approach can deliver new color-tunable RTP materials for applications including multicolor display, information encryption, and gas detection.
Keywords: charge transfer; color‐tunable; non‐aromatic polymer; quinoline zwitterion; room‐temperature phosphorescence.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Achieving Tunable Organic Afterglow and UV-Irradiation-Responsive Ultralong Room-Temperature Phosphorescence from Pyridine-Substituted Triphenylamine Derivatives.Adv Mater. 2023 Jul;35(28):e2301874. doi: 10.1002/adma.202301874. Epub 2023 May 30. Adv Mater. 2023. PMID: 37026437
-
Construction of starch-based room temperature phosphorescence materials with wide color-tunable long afterglow and even persistent near-infrared luminescence via Förster resonance energy transfer.Int J Biol Macromol. 2025 Jan;284(Pt 2):138175. doi: 10.1016/j.ijbiomac.2024.138175. Epub 2024 Nov 28. Int J Biol Macromol. 2025. PMID: 39615724
-
Achieving Colorful Ultralong-Lifetime Room-Temperature Phosphorescence Based on Benzocarbazole Derivatives through Resonance Energy Transfer.ACS Appl Mater Interfaces. 2024 May 15;16(19):25415-25421. doi: 10.1021/acsami.4c04921. Epub 2024 May 2. ACS Appl Mater Interfaces. 2024. PMID: 38696539
-
Long-Lived Dynamic Room Temperature Phosphorescence from Carbon Dots Based Materials.Small. 2023 Aug;19(31):e2206429. doi: 10.1002/smll.202206429. Epub 2023 Jan 6. Small. 2023. PMID: 36609989 Review.
-
Unveiling the potential of triphenylphosphine salts in tuning organic room temperature phosphorescence.Chem Commun (Camb). 2024 Aug 27;60(70):9328-9339. doi: 10.1039/d4cc03156c. Chem Commun (Camb). 2024. PMID: 39113543 Review.
References
-
- a) Baldo M. A., O'Brien D. F., You Y., Shoustikov A., Sibley S., Thompson M. E., Forrest S. R., Nature 1998, 395, 151;
- b) Bolton O., Lee K., Kim H.‐J., Lin K. Y., Kim J., Nat. Chem. 2011, 3, 205. - PubMed
-
- a) Ju H., Zhang H., Hou L. X., Zuo M., Du M., Huang F., Zheng Q., Wu Z. L., J. Am. Chem. Soc. 2023, 145, 3763; - PubMed
- b) Tang M., Wen J., Sun Y., Hou Q., Cai X., He W., Xie X., Ding H., Li F., Zheng L., Shi Y., Cao Q., Adv. Funct. Mater. 2024, 34, 2314130.
-
- Zhang Y., Chen X., Xu J., Zhang Q., Gao L., Wang Z., Qu L., Wang K., Li Y., Cai Z., Zhao Y., Yang C., J. Am. Chem. Soc. 2022, 144, 6107. - PubMed
-
- a) Wan K., Zhai Y., Liu S., Li J., Li S., Strehmel B., Chen Z., James T. D., Angew. Chem., Int. Ed. 2022, 61, e202202760; - PubMed
- b) Bi X., Shi Y., Peng T., Yue S., Wang F., Zheng L., Cao Q. E., Adv. Funct. Mater. 2021, 31, 2101312;
- c) Yue S., Ding H., Sun Y., Tang M., Wen J., Peng Y., Zheng L., Wang F., Shi Y., Cao Q., J. Phys. Chem. Lett. 2022, 13, 10190. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
