Controlling Pericellular Oxygen Tension in Cell Culture Reveals Distinct Breast Cancer Responses to Low Oxygen Tensions
- PMID: 38874400
- PMCID: PMC11321643
- DOI: 10.1002/advs.202402557
Controlling Pericellular Oxygen Tension in Cell Culture Reveals Distinct Breast Cancer Responses to Low Oxygen Tensions
Abstract
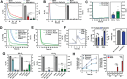
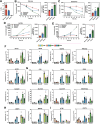
In oxygen (O2)-controlled cell culture, an indispensable tool in biological research, it is presumed that the incubator setpoint equals the O2 tension experienced by cells (i.e., pericellular O2). However, it is discovered that physioxic (5% O2) and hypoxic (1% O2) setpoints regularly induce anoxic (0% O2) pericellular tensions in both adherent and suspension cell cultures. Electron transport chain inhibition ablates this effect, indicating that cellular O2 consumption is the driving factor. RNA-seq analysis revealed that primary human hepatocytes cultured in physioxia experience ischemia-reperfusion injury due to cellular O2 consumption. A reaction-diffusion model is developed to predict pericellular O2 tension a priori, demonstrating that the effect of cellular O2 consumption has the greatest impact in smaller volume culture vessels. By controlling pericellular O2 tension in cell culture, it is found that hypoxia vs. anoxia induce distinct breast cancer transcriptomic and translational responses, including modulation of the hypoxia-inducible factor (HIF) pathway and metabolic reprogramming. Collectively, these findings indicate that breast cancer cells respond non-monotonically to low O2, suggesting that anoxic cell culture is not suitable for modeling hypoxia. Furthermore, it is shown that controlling atmospheric O2 tension in cell culture incubators is insufficient to regulate O2 in cell culture, thus introducing the concept of pericellular O2-controlled cell culture.
Keywords: anoxia; breast cancer; cancer metabolism; cell culture; hypoxia; hypoxia‐inducible factors; oxygen; physioxia.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Update of
-
Controlling pericellular oxygen tension in cell culture reveals distinct breast cancer responses to low oxygen tensions.bioRxiv [Preprint]. 2023 Oct 3:2023.10.02.560369. doi: 10.1101/2023.10.02.560369. bioRxiv. 2023. Update in: Adv Sci (Weinh). 2024 Aug;11(30):e2402557. doi: 10.1002/advs.202402557. PMID: 37873449 Free PMC article. Updated. Preprint.
Similar articles
-
Controlling pericellular oxygen tension in cell culture reveals distinct breast cancer responses to low oxygen tensions.bioRxiv [Preprint]. 2023 Oct 3:2023.10.02.560369. doi: 10.1101/2023.10.02.560369. bioRxiv. 2023. Update in: Adv Sci (Weinh). 2024 Aug;11(30):e2402557. doi: 10.1002/advs.202402557. PMID: 37873449 Free PMC article. Updated. Preprint.
-
Oxygen control in cell culture - Your cells may not be experiencing what you think!Free Radic Biol Med. 2025 Jan;226:279-287. doi: 10.1016/j.freeradbiomed.2024.11.036. Epub 2024 Nov 20. Free Radic Biol Med. 2025. PMID: 39577817 Review.
-
The effect of baseline O2 conditions on the response of prostate cancer cells to hypoxia.Am J Physiol Cell Physiol. 2024 Jul 1;327(1):C97-C112. doi: 10.1152/ajpcell.00155.2024. Epub 2024 Apr 22. Am J Physiol Cell Physiol. 2024. PMID: 38646786
-
Pericellular oxygen monitoring with integrated sensor chips for reproducible cell culture experiments.Cell Prolif. 2014 Apr;47(2):180-8. doi: 10.1111/j.1365-2184.2013.12089.x. Epub 2014 Jan 25. Cell Prolif. 2014. PMID: 24460744 Free PMC article.
-
Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research.Free Radic Biol Med. 2017 Dec;113:311-322. doi: 10.1016/j.freeradbiomed.2017.10.003. Epub 2017 Oct 13. Free Radic Biol Med. 2017. PMID: 29032224 Free PMC article. Review.
Cited by
-
Breaking barriers: Smart vaccine platforms for cancer immunomodulation.Cancer Commun (Lond). 2025 May;45(5):529-571. doi: 10.1002/cac2.70002. Epub 2025 Feb 3. Cancer Commun (Lond). 2025. PMID: 39901621 Free PMC article. Review.
-
Limited oxygen in standard cell culture alters metabolism and function of differentiated cells.EMBO J. 2024 Jun;43(11):2127-2165. doi: 10.1038/s44318-024-00084-7. Epub 2024 Apr 5. EMBO J. 2024. PMID: 38580776 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
