Phase I/II clinical trial of brentuximab vedotin for pretreated Japanese patients with CD30-positive cutaneous T-cell lymphoma
- PMID: 38874430
- PMCID: PMC11483954
- DOI: 10.1111/1346-8138.17324
Phase I/II clinical trial of brentuximab vedotin for pretreated Japanese patients with CD30-positive cutaneous T-cell lymphoma
Abstract
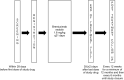
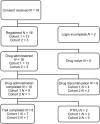
Brentuximab vedotin (BV), a conjugate of anti-CD30 antibody and monomethyl auristatin E, has emerged as a promising treatment option for refractory CD30+ mycosis fungoides (MF) and primary cutaneous anaplastic large-cell lymphoma (pcALCL). BV has been shown to be safe and effective in treating Hodgkin's lymphoma and peripheral T-cell lymphoma. This multicenter, prospective, single-arm phase I/II study evaluated the efficacy of BV in Japanese patients with CD30+ cutaneous lymphomas, namely CD30+ cutaneous T-cell lymphoma. Participants were divided into two groups: those with CD30+ MF or pcALCL (cohort 1, n = 13) and those with CD30+ lymphoproliferative disorders other than those in cohort 1 (cohort 2, n = 3). The studied population included the full analysis set (FAS), modified FAS (mFAS), and safety analysis set (SAF). These sets were identified in cohorts 1 and 1 + 2 and labeled FAS1 and FAS2, mFAS1 and mFAS2, and SAF1 and SAF2, respectively. Each treatment cycle lasted 3 weeks, and BV was continued for up to 16 cycles after the third cycle based on treatment response. The primary endpoint was the 4-month objective response rate (ORR4) determined by the Independent Review Forum (IRF). ORR4 was 69.2% for FAS1 and 62.5% for FAS2 (P < 0.0001). Secondary endpoints of ORR, assessed using the global response score (53.8% in FAS1) and modified severity-weighted assessment tool (62.5% in FAS1), using the IRF, provided results comparable to the primary findings. The incidence of ≥grade 3 adverse events (≥15%) in SAF1 was peripheral neuropathy in three patients (23%) and fever and eosinophilia in two patients (15%). In conclusion, BV showed favorable efficacy, tolerability, and safety profile in Japanese patients with relapsed or refractory CD30+ primary cutaneous T-cell lymphoma. The trial was registered with University Hospital Medical Information Network Clinical Trials Registry, Japan (protocol ID: UMIN000034205).
Keywords: anaplastic large‐cell lymphoma; brentuximab vedotin; cutaneous T‐cell lymphoma; mycosis fungoides; peripheral T‐cell lymphoma.
© 2024 The Author(s). The Journal of Dermatology published by John Wiley & Sons Australia, Ltd on behalf of Japanese Dermatological Association.
Conflict of interest statement
Yoji Hirai received research grants, consultancy fees, speaker fees, and funds for staff members from Takeda Pharmaceutical Company Limited. Tomomitsu Miyagaki, Riichiro Abe, and Shin Morizane are members of Editorial Board of the
Figures


References
-
- Fujii K, Hamada T, Shimauchi T, Asai J, Fujisawa Y, Ihn H, et al. Cutaneous lymphoma in Japan, 2012–2017: a nationwide study. J Dermatol Sci. 2020;97:187–193. - PubMed
-
- Olsen EA, Whittaker S, Kim YH, Duvic M, Prince HM, Lessin SR, et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organization for Research and Treatment of Cancer. J Clin Oncol. 2011;29:2598–2607. - PMC - PubMed
-
- Agar NS, Wedgeworth E, Crichton S, Mitchell TJ, Cox M, Ferreira S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28:4730–4739. - PubMed
-
- Suzuki S, Ito K, Ito M, Kawai K. Prognosis of 100 Japanese patients with mycosis fungoides and Sézary syndrome. J Dermatol Sci. 2010;57:37–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

