Hsp90 Promotes Gastric Cancer Cell Metastasis and Stemness by Regulating the Regional Distribution of Glycolysis-Related Metabolic Enzymes in the Cytoplasm
- PMID: 38874476
- PMCID: PMC11434123
- DOI: 10.1002/advs.202310109
Hsp90 Promotes Gastric Cancer Cell Metastasis and Stemness by Regulating the Regional Distribution of Glycolysis-Related Metabolic Enzymes in the Cytoplasm
Abstract
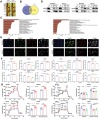
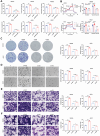
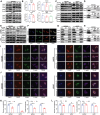
Heat-shock protein 90 (Hsp90) plays a crucial role in tumorigenesis and tumor progression; however, its mechanism of action in gastric cancer (GC) remains unclear. Here, the role of Hsp90 in GC metabolism is the focus of this research. High expression of Hsp90 in GC tissues can interact with glycolysis, collectively affecting prognosis in clinical samples. Both in vitro and in vivo experiments demonstrate that Hsp90 is able to regulate the migration and stemness properties of GC cells. Metabolic phenotype analyses indicate that Hsp90 influences glycolytic metabolism. Mechanistically, Hsp90 interacts with glycolysis-related enzymes, forming multienzyme complexes to enhance glycolysis efficiency and yield. Additionally, Hsp90 binds to cytoskeleton-related proteins, regulating the regional distribution of glycolytic enzymes at the cell margin and lamellar pseudopods. This effect could lead to a local increase in efficient energy supply from glycolysis, further promoting epithelial-mesenchymal transition (EMT) and metastasis. In summary, Hsp90, through its interaction with metabolic enzymes related to glycolysis, forms multi-enzyme complexes and regulates regional distribution of glycolysis by dynamic cytoskeletal adjustments, thereby promoting the migration and stemness of GC cells. These conclusions also support the potential for a combined targeted approach involving Hsp90, glycolysis, and the cytoskeleton in clinical therapy.
Keywords: Hsp90; combination therapy; glycolysis; multienzyme complex; regionalized distribution.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
