Sensory Nerve Regulation via H3K27 Demethylation Revealed in Akermanite Composite Microspheres Repairing Maxillofacial Bone Defect
- PMID: 38874525
- PMCID: PMC11321702
- DOI: 10.1002/advs.202400242
Sensory Nerve Regulation via H3K27 Demethylation Revealed in Akermanite Composite Microspheres Repairing Maxillofacial Bone Defect
Abstract
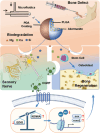
Maxillofacial bone defects exhibit intricate anatomy and irregular morphology, presenting challenges for effective treatment. This study aimed to address these challenges by developing an injectable bioactive composite microsphere, termed D-P-Ak (polydopamine-PLGA-akermanite), designed to fit within the defect site while minimizing injury. The D-P-Ak microspheres biodegraded gradually, releasing calcium, magnesium, and silicon ions, which, notably, not only directly stimulated the osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) but also activated sensory nerve cells to secrete calcitonin gene-related peptide (CGRP), a key factor in bone repair. Moreover, the released CGRP enhanced the osteogenic differentiation of BMSCs through epigenetic methylation modification. Specifically, inhibition of EZH2 and enhancement of KDM6A reduced the trimethylation level of histone 3 at lysine 27 (H3K27), thereby activating the transcription of osteogenic genes such as Runx2 and Osx. The efficacy of the bioactive microspheres in bone repair is validated in a rat mandibular defect model, demonstrating that peripheral nerve response facilitates bone regeneration through epigenetic modification. These findings illuminated a novel strategy for constructing neuroactive osteo-inductive biomaterials with potential for further clinical applications.
Keywords: bioactive ions; bone regeneration; composite microspheres; methylation modification; sensory nerve.
© 2024 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Injectable PLGA microspheres with tunable magnesium ion release for promoting bone regeneration.Acta Biomater. 2019 Feb;85:294-309. doi: 10.1016/j.actbio.2018.12.017. Epub 2018 Dec 13. Acta Biomater. 2019. PMID: 30553873
-
Microsphere-Gel Composite System with Mesenchymal Stem Cell Recruitment, Antibacterial, and Immunomodulatory Properties Promote Bone Regeneration via Sequential Release of LL37 and W9 Peptides.ACS Appl Mater Interfaces. 2022 Aug 31;14(34):38525-38540. doi: 10.1021/acsami.2c10242. Epub 2022 Aug 16. ACS Appl Mater Interfaces. 2022. PMID: 35973165
-
Repair of Calvarial Bone Defect Using Jarid1a-Knockdown Bone Mesenchymal Stem Cells in Rats.Tissue Eng Part A. 2018 May;24(9-10):711-718. doi: 10.1089/ten.tea.2017.0168. Epub 2017 Oct 19. Tissue Eng Part A. 2018. PMID: 28903624
-
A Naringin-loaded gelatin-microsphere/nano-hydroxyapatite/silk fibroin composite scaffold promoted healing of critical-size vertebral defects in ovariectomised rat.Int J Biol Macromol. 2021 Dec 15;193(Pt A):510-518. doi: 10.1016/j.ijbiomac.2021.10.036. Epub 2021 Oct 25. Int J Biol Macromol. 2021. PMID: 34710477
-
A sericin/ graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone.Theranostics. 2020 Jan 1;10(2):741-756. doi: 10.7150/thno.39502. eCollection 2020. Theranostics. 2020. PMID: 31903148 Free PMC article.
Cited by
-
Bioactive Inorganic Materials for Innervated Multi-Tissue Regeneration.Adv Sci (Weinh). 2025 Apr;12(13):e2415344. doi: 10.1002/advs.202415344. Epub 2025 Feb 27. Adv Sci (Weinh). 2025. PMID: 40013907 Free PMC article. Review.
-
Strategies for promoting neurovascularization in bone regeneration.Mil Med Res. 2025 Mar 3;12(1):9. doi: 10.1186/s40779-025-00596-1. Mil Med Res. 2025. PMID: 40025573 Free PMC article. Review.
References
-
- a) Costela‐Ruiz V. J., Melguizo‐Rodriguez L., Bellotti C., Illescas‐Montes R., Stanco D., Arciola C. R., Lucarelli E., Int. J. Mol. Sci. 2022, 23; - PMC - PubMed
- b) Kang Y., Xu C., Meng L., Dong X., Qi M., Jiang D., Bioact Mater. 2022, 18, 26; - PMC - PubMed
- c) He Y., Wang W., Lin S., Yang Y., Song L., Jing Y., Chen L., He Z., Li W., Xiong A., Yeung K. W. K., Zhao Q., Jiang Y., Li Z., Pei G., Zhang Z. Y., Bioact. Mater. 2022, 9, 491; - PMC - PubMed
- d) Silva M. M., Cyster L. A., Barry J. J., Yang X. B., Oreffo R. O., Grant D. M., Scotchford C. A., Howdle S. M., Shakesheff K. M., Rose F. R., Biomaterials 2006, 27, 5909. - PubMed
-
- a) Haidar Z. S., Hamdy R. C., Tabrizian M., Biotechnol. Lett. 2009, 31, 1817; - PubMed
- b) Kim D.‐S., Lee J.‐K., Kim Jun H., Lee J., Kim Dong S., An S., Park S.‐B., Kim T.‐H., Rim Jong S., Lee S., Han Dong K., Sci. Adv. 2021, 7, eabj1083; - PMC - PubMed
- c) Fang T., Yuan Z., Zhao Y., Li X., Zhai Y., Li J., Wang X., Rao N., Ge L., Cai Q., Chem. Eng. J. 2019, 370, 573.
-
- Cai Z., Jiang H., Lin T., Wang C., Ma J., Gao R., Jiang Y., Zhou X., Mater. Today Adv. 2022, 16, 100315.
MeSH terms
Substances
Grants and funding
- 21YF1423800/Shanghai Sailing Program
- CSA-O2022-10/Young Clinical Research Fund of the Chinese Stomatological Association
- 2022SKLS-KFKT009/Opening Research fund from Shanghai Key Laboratory of Stomatology
- 2020 Talent Cultivation Project of The 9th People's Hospital
- JC202002/Shanghai Jiao Tong University School of Medicine
LinkOut - more resources
Full Text Sources
Research Materials
