Cellular and Molecular Insights into the Divergence of Neural Stem Cells on Matrigel and Poly-l-lysine Interfaces
- PMID: 38874539
- PMCID: PMC11212020
- DOI: 10.1021/acsami.4c02575
Cellular and Molecular Insights into the Divergence of Neural Stem Cells on Matrigel and Poly-l-lysine Interfaces
Abstract
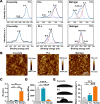
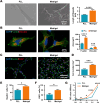
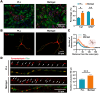
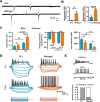
Poly-l-lysine (PLL) and Matrigel, both classical coating materials for culture substrates in neural stem cell (NSC) research, present distinct interfaces whose effect on NSC behavior at cellular and molecular levels remains ambiguous. Our investigation reveals intriguing disparities: although both PLL and Matrigel interfaces are hydrophilic and feature amine functional groups, Matrigel stands out with lower stiffness and higher roughness. Based on this diversity, Matrigel surpasses PLL, driving NSC adhesion, migration, and proliferation. Intriguingly, PLL promotes NSC differentiation into astrocytes, whereas Matrigel favors neural differentiation and the physiological maturation of neurons. At the molecular level, Matrigel showcases a wider upregulation of genes linked to NSC behavior. Specifically, it enhances ECM-receptor interaction, activates the YAP transcription factor, and heightens glycerophospholipid metabolism, steering NSC proliferation and neural differentiation. Conversely, PLL upregulates genes associated with glial cell differentiation and amino acid metabolism and elevates various amino acid levels, potentially linked to its support for astrocyte differentiation. These distinct transcriptional and metabolic activities jointly shape the divergent NSC behavior on these substrates. This study significantly advances our understanding of substrate regulation on NSC behavior, offering novel insights into optimizing and targeting the application of these surface coating materials in NSC research.
Keywords: Differentiation; Electrophysiological function; Matrigel; Metabolomics; Neural stem cell; Poly-l-lysine; YAP.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Systematic Evaluation of Extracellular Coating Matrix on the Differentiation of Human-Induced Pluripotent Stem Cells to Cortical Neurons.Int J Mol Sci. 2024 Dec 30;26(1):230. doi: 10.3390/ijms26010230. Int J Mol Sci. 2024. PMID: 39796088 Free PMC article.
-
Optimal poly(L-lysine) grafting density in hydrogels for promoting neural progenitor cell functions.Biomacromolecules. 2012 May 14;13(5):1663-74. doi: 10.1021/bm300381d. Epub 2012 May 3. Biomacromolecules. 2012. PMID: 22533450 Free PMC article.
-
Surface coating as a key parameter in engineering neuronal network structures in vitro.Biointerphases. 2012 Dec;7(1-4):29. doi: 10.1007/s13758-012-0029-7. Epub 2012 Apr 24. Biointerphases. 2012. PMID: 22589072
-
The Effects of Matrigel® on the Survival and Differentiation of a Human Neural Progenitor Dissociated Sphere Culture.Anat Rec (Hoboken). 2020 Mar;303(3):441-450. doi: 10.1002/ar.24131. Epub 2019 May 8. Anat Rec (Hoboken). 2020. PMID: 30968577
-
Comparative surface energetic study of Matrigel® and collagen I interactions with endothelial cells.Colloids Surf B Biointerfaces. 2017 Jul 1;155:71-82. doi: 10.1016/j.colsurfb.2017.04.004. Epub 2017 Apr 4. Colloids Surf B Biointerfaces. 2017. PMID: 28411477
Cited by
-
YAP Signaling in Glia: Pivotal Roles in Neurological Development, Regeneration and Diseases.Neurosci Bull. 2025 Mar;41(3):501-519. doi: 10.1007/s12264-024-01308-w. Epub 2024 Nov 6. Neurosci Bull. 2025. PMID: 39503968 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

