PlexinB1 Inactivation Reprograms Immune Cells in the Tumor Microenvironment, Inhibiting Breast Cancer Growth and Metastatic Dissemination
- PMID: 38874583
- PMCID: PMC11369622
- DOI: 10.1158/2326-6066.CIR-23-0289
PlexinB1 Inactivation Reprograms Immune Cells in the Tumor Microenvironment, Inhibiting Breast Cancer Growth and Metastatic Dissemination
Abstract
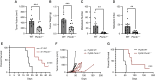
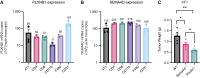
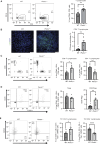
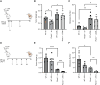
Semaphorin-plexin signaling plays a major role in the tumor microenvironment (TME). In particular, Semaphorin 4D (SEMA4D) has been shown to promote tumor growth and metastasis; however, the role of its high-affinity receptor Plexin-B1 (PLXNB1), which is expressed in the TME, is poorly understood. In this study, we directly targeted PLXNB1 in the TME of triple-negative murine breast carcinoma to elucidate its relevance in cancer progression. We found that primary tumor growth and metastatic dissemination were strongly reduced in PLXNB1-deficient mice, which showed longer survival. PLXNB1 loss in the TME induced a switch in the polarization of tumor-associated macrophages (TAM) toward a pro-inflammatory M1 phenotype and enhanced the infiltration of CD8+ T lymphocytes both in primary tumors and in distant metastases. Moreover, PLXNB1 deficiency promoted a shift in the Th1/Th2 balance of the T-cell population and an antitumor gene signature, with the upregulation of Icos, Perforin-1, Stat3, and Ccl5 in tumor-infiltrating lymphocytes (TILs). We thus tested the translational relevance of TME reprogramming driven by PLXNB1 inactivation for responsiveness to immunotherapy. Indeed, in the absence of PLXNB1, the efficacy of anti-PD-1 blockade was strongly enhanced, efficiently reducing tumor growth and distant metastasis. Consistent with this, pharmacological PLXNB1 blockade by systemic treatment with a specific inhibitor significantly hampered breast cancer growth and enhanced the antitumor activity of the anti-PD-1 treatment in a preclinical model. Altogether, these data indicate that PLXNB1 signaling controls the antitumor immune response in the TME and highlight this receptor as a promising immune therapeutic target for metastatic breast cancers.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
J. Takagi reports grants from Japan Agency for Medical Research and Development (AMED) during the conduct of the study and has a patent for WO2019026920A1 issued, licensed, and with royalties paid from MiraBiologics Inc. Dr. J. Takagi is a cofounder and shareholder of MiraBiologics Inc. No disclosures were reported by the other authors.
Figures


References
-
- Coughlin SS. Epidemiology of breast cancer in women. Adv Exp Med Biol 2019;1152:9–29. - PubMed
-
- de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell 2023;41:374–403. - PubMed
-
- Duan Q, Zhang H, Zheng J, Zhang L. Turning cold into hot: firing up the tumor microenvironment. Trends Cancer 2020;6:605–18. - PubMed
-
- de Melo Gagliato D, Buzaid AC, Perez-Garcia J, Cortes J. Immunotherapy in breast cancer: current practice and clinical challenges. BioDrugs 2020;34:611–23. - PubMed
MeSH terms
Substances
Grants and funding
- IG # 19957/Fondazione AIRC per la ricerca sul cancro ETS (AIRC)
- PTCRC-INTRA 2020 - SEE- HER/Fondazione del Piemonte per L'Oncologia (FPRC)
- Ricerca Corrente 2022-23/Ministero della Salute (Italy Ministry of Health)
- IG # 19923/Fondazione AIRC per la ricerca sul cancro ETS (AIRC)
- Ricerca Corrente 2022-23/Ministero della Salute (Italy Ministry of Health)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

