Human umbilical cord-derived mesenchymal stem cells attenuate hepatic stellate cells activation and liver fibrosis
- PMID: 38874773
- PMCID: PMC11178641
- DOI: 10.1007/s11033-024-09664-6
Human umbilical cord-derived mesenchymal stem cells attenuate hepatic stellate cells activation and liver fibrosis
Abstract
Background: Liver cirrhosis, a prevalent chronic liver disease, is characterized by liver fibrosis as its central pathological process. Recent advancements highlight the clinical efficacy of umbilical cord mesenchymal stem cell (UC-MSC) therapy in the treatment of liver cirrhosis.
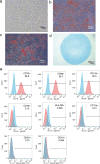
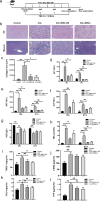
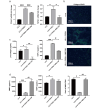
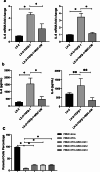
Methods and results: We investigated the pharmacodynamic effects of UC-MSCs and MSC conditional medium (MSC-CM) in vivo, utilizing a carbon tetrachloride (CCl4)-induced fibrotic rat model. Concurrently, we assessed the in vitro impact of MSCs and MSC-CM on various cellular process of hepatic stellate cells (HSCs), including proliferation, apoptosis, activation, immunomodulatory capabilities, and inflammatory factor secretion. Our results indicate that both MSCs and MSC-CM significantly ameliorate the pathological extent of fibrosis in animal tissues, reducing the collagen content, serum biochemical indices and fibrosis biomarkers. In vitro, MSC-CM significantly inhibited the activation of the HSC line LX-2. Notably, MSC-CM modulated the expression of type I procollagen and TGFβ-1 while increasing MMP1 expression. This modulation restored the MMP1/TIMP1 ratio imbalance and extracellular matrix deposition in TGFβ-1 induced fibrosis. Both MSCs and MSC-CM not only induced apoptosis in HSCs but also suppressed proliferation and inflammatory cytokine release from activated HSCs. Furthermore, MSCs and MSC-CM exerted a suppressive effect on total lymphocyte activation.
Conclusions: UC-MSCs and MSC-CM primarily modulate liver fibrosis severity by regulating HSC activation. This study provides both in vivo and in vitro pharmacodynamic evidence supporting the use of MSCs in liver fibrosis treatment.
Keywords: CCL4-induced liver fibrosis; Hepatic stellate cell activation; Immunomodulation; MSC conditional medium; UC-MSC.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

