Neuroanatomical evidence and a mouse calcitonin gene-related peptide model in line with human functional magnetic resonance imaging data support the involvement of peptidergic Edinger-Westphal nucleus in migraine
- PMID: 38875125
- PMCID: PMC11562765
- DOI: 10.1097/j.pain.0000000000003294
Neuroanatomical evidence and a mouse calcitonin gene-related peptide model in line with human functional magnetic resonance imaging data support the involvement of peptidergic Edinger-Westphal nucleus in migraine
Abstract
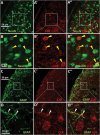
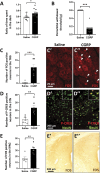
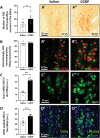
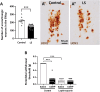
The urocortin 1 (UCN1)-expressing centrally projecting Edinger-Westphal (EWcp) nucleus is influenced by circadian rhythms, hormones, stress, and pain, all known migraine triggers. Our study investigated EWcp's potential involvement in migraine. Using RNAscope in situ hybridization and immunostaining, we examined the expression of calcitonin gene-related peptide (CGRP) receptor components in both mouse and human EWcp and dorsal raphe nucleus (DRN). Tracing study examined connection between EWcp and the spinal trigeminal nucleus (STN). The intraperitoneal CGRP injection model of migraine was applied and validated by light-dark box, and von Frey assays in mice, in situ hybridization combined with immunostaining, were used to assess the functional-morphological changes. The functional connectivity matrix of EW was examined using functional magnetic resonance imaging in control humans and interictal migraineurs. We proved the expression of CGRP receptor components in both murine and human DRN and EWcp. We identified a direct urocortinergic projection from EWcp to the STN. Photophobic behavior, periorbital hyperalgesia, increased c-fos gene-encoded protein immunoreactivity in the lateral periaqueductal gray matter and trigeminal ganglia, and phosphorylated c-AMP-responsive element binding protein in the STN supported the efficacy of CGRP-induced migraine-like state. Calcitonin gene-related peptide administration also increased c-fos gene-encoded protein expression, Ucn1 mRNA, and peptide content in EWcp/UCN1 neurons while reducing serotonin and tryptophan hydroxylase-2 levels in the DRN. Targeted ablation of EWcp/UCN1 neurons induced hyperalgesia. A positive functional connectivity between EW and STN as well as DRN has been identified by functional magnetic resonance imaging. The presented data strongly suggest the regulatory role of EWcp/UCN1 neurons in migraine through the STN and DRN with high translational value.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the International Association for the Study of Pain.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
All authors read and approved the final version of the manuscript.
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Figures






References
-
- Asghar MS, Hansen AE, Amin FM, van der Geest RJ, Koning Pvd, Larsson HBW, Olesen J, Ashina M. Evidence for a vascular factor in migraine. Ann Neurol 2011;69:635–45. - PubMed
-
- Ashina M, Hansen JM, Á Dunga BO, Olesen J. Human models of migraine—short-term pain for long-term gain. Nat Rev Neurol 2017;13:713–24. - PubMed
-
- Ashina H, Schytz HW, Ashina M. CGRP in human models of migraine. Handb Exp Pharmacol 2019;255:109–20. - PubMed
-
- Avona A, Mason BN, Lackovic J, Wajahat N, Motina M, Quigley L, Burgos-Vega C, Moldovan Loomis C, Garcia-Martinez LF, Akopian AN, Price TJ, Dussor G. Repetitive stress in mice causes migraine-like behaviors and calcitonin gene-related peptide-dependent hyperalgesic priming to a migraine trigger. PAIN 2020;161:2539–50. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

