Differential phosphorylation of Ca2+-permeable channel CYCLIC NUCLEOTIDE-GATED CHANNEL20 modulates calcium-mediated freezing tolerance in Arabidopsis
- PMID: 38875155
- PMCID: PMC11449002
- DOI: 10.1093/plcell/koae177
Differential phosphorylation of Ca2+-permeable channel CYCLIC NUCLEOTIDE-GATED CHANNEL20 modulates calcium-mediated freezing tolerance in Arabidopsis
Abstract
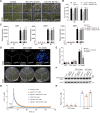
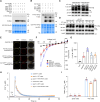
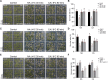
Plants respond to cold stress at multiple levels, including increasing cytosolic calcium (Ca2+) influx and triggering the expression of cold-responsive genes. In this study, we show that the Ca2+-permeable channel CYCLIC NUCLEOTIDE-GATED CHANNEL20 (CNGC20) positively regulates freezing tolerance in Arabidopsis (Arabidopsis thaliana) by mediating cold-induced Ca2+ influx. Moreover, we demonstrate that the leucine-rich repeat receptor-like kinase PLANT PEPTIDE CONTAINING SULFATED TYROSINE1 RECEPTOR (PSY1R) is activated by cold, phosphorylating and enhancing the activity of CNGC20. The psy1r mutant exhibits decreased cold-evoked Ca2+ influx and freezing tolerance. Conversely, COLD-RESPONSIVE PROTEIN KINASE1 (CRPK1), a protein kinase that negatively regulates cold signaling, phosphorylates and facilitates the degradation of CNGC20 under prolonged periods of cold treatment, thereby attenuating freezing tolerance. This study thus identifies PSY1R and CRPK1 kinases that regulate CNGC20 activity and stability, respectively, thereby antagonistically modulating freezing tolerance in plants.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

